ABC51
limited activity against PPT1 in situ or in vitro, but was a good inhibitor of ABHD4 in vitro (IC50 value of 0.5 microM) as measured with an N-acyl phosphatidylethanolamine (NAPE) substrate hydrolysis assay performed with lysates from ABHD4-transfected HEK293T cells
General
Type : Carbamate, Piperidine, N-hydroxyhydantoin, Phenoxyphenyl
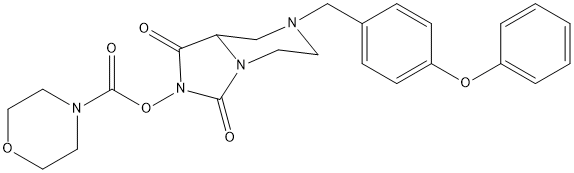
Chemical_Nomenclature : [1,3-dioxo-7-[(4-phenoxyphenyl)methyl]-5,6,8,8a-tetrahydroimidazo[1,5-a]pyrazin-2-yl] morpholine-4-carboxylate
Canonical SMILES : C1CN2C(CN1CC3=CC=C(C=C3)OC4=CC=CC=C4)C(=O)N(C2=O)OC(=O)N5CCOCC5
InChI : InChI=1S\/C24H26N4O6\/c29-22-21-17-25(16-18-6-8-20(9-7-18)33-19-4-2-1-3-5-19)10-11-27(21)23(30)28(22)34-24(31)26-12-14-32-15-13-26\/h1-9,21H,10-17H2
InChIKey : NZPCUYXTRLSQCQ-UHFFFAOYSA-N
Other name(s) : SCHEMBL17286394
MW : 466.5
Formula : C24H26N4O6
CAS_number :
PubChem : 118535304
UniChem : NZPCUYXTRLSQCQ-UHFFFAOYSA-N
Target
Families : ABC51 ligand of proteins in family
CGI-58_ABHD5_ABHD4
Protein :
human-ABHD4
References (3)
| Title : Selective N-Hydroxyhydantoin Carbamate Inhibitors of Mammalian Serine Hydrolases - Cognetta_2015_Chem.Biol_22_928 |
| Author(s) : Cognetta AB, 3rd , Niphakis MJ , Lee HC , Martini ML , Hulce JJ , Cravatt BF |
| Ref : Chemical Biology , 22 :928 , 2015 |
| Abstract : |
| PubMedSearch : Cognetta_2015_Chem.Biol_22_928 |
| PubMedID: 26120000 |
| Title : Selective N-Hydroxyhydantoin Carbamate Inhibitors of Mammalian Serine Hydrolases - Cognetta_2015_Chem.Biol_22_928 |
| Author(s) : Cognetta AB, 3rd , Niphakis MJ , Lee HC , Martini ML , Hulce JJ , Cravatt BF |
| Ref : Chemical Biology , 22 :928 , 2015 |
| Abstract : |
| PubMedSearch : Cognetta_2015_Chem.Biol_22_928 |
| PubMedID: 26120000 |
| Title : Selective N-Hydroxyhydantoin Carbamate Inhibitors of Mammalian Serine Hydrolases - Cognetta_2015_Chem.Biol_22_928 |
| Author(s) : Cognetta AB, 3rd , Niphakis MJ , Lee HC , Martini ML , Hulce JJ , Cravatt BF |
| Ref : Chemical Biology , 22 :928 , 2015 |
| Abstract : |
| PubMedSearch : Cognetta_2015_Chem.Biol_22_928 |
| PubMedID: 26120000 |