ARUK3005522
General
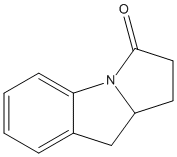
Type : Fragment inhibitor of Notum, Indole
Chemical_Nomenclature : (3~{a}~{R})-2,3,3~{a},4-tetrahydropyrrolo[1,2-a]indol-1-one
Canonical SMILES : c1ccc2c(c1)C[C@@H]3N2C(=O)CC3 || C1CC(=O)N2C1CC3=CC=CC=C32
InChI : InChI=1S\/C11H11NO\/c13-11-6-5-9-7-8-3-1-2-4-10(8)12(9)11\/h1-4,9H,5-7H2\/t9-\/m1\/s1 || InChI=1S\/C11H11NO\/c13-11-6-5-9-7-8-3-1-2-4-10(8)12(9)11\/h1-4,9H,5-7H2
InChIKey : QPPMCKRHXDXQPA-SECBINFHSA-N || QPPMCKRHXDXQPA-UHFFFAOYSA-N
Other name(s) : RGU || Compound 5f || 1,2,9,9a-tetrahydro-3H-pyrrolo[1,2-a]indol-3-one || 3,3a,4,9-tetrahydro-2H-pyrrolo[1,5-a]indol-1-one || 1,2,9,9a-Tetrahydro-3H-pyrrolo[1,2-a]indole-3-one || 2,3,3a,4-tetrahydropyrrolo[1,2-a]indol-1-one
MW : 173.21
Formula : C11H11NO
CAS_number :
UniChem : QPPMCKRHXDXQPA-SECBINFHSA-N, QPPMCKRHXDXQPA-UHFFFAOYSA-N
Target
Families : ARUK3005522 ligand of proteins in family
Pectinacetylesterase-Notum
Structure :
8BSZ
Protein :
human-NOTUM
References (1)
| Title : Designed switch from covalent to non-covalent inhibitors of carboxylesterase Notum activity - Atkinson_2023_Eur.J.Med.Chem_251_115132 |
| Author(s) : Atkinson BN , Willis NJ , Zhao Y , Patel C , Frew S , Costelloe K , Magno L , Svensson F , Jones EY , Fish PV |
| Ref : Eur Journal of Medicinal Chemistry , 251 :115132 , 2023 |
| Abstract : |
| PubMedSearch : Atkinson_2023_Eur.J.Med.Chem_251_115132 |
| PubMedID: 36934521 |
| Gene_locus related to this paper: human-NOTUM |