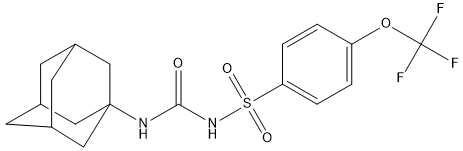
Adamantyl-trifluoromethoxy-phenyl-sulfonylurea-4f
Human sEH Inhibition IC50 1.69 nM; Mouse sEH Inhibition IC50 0.29 microM . 4f showed anti-inflammatory efficacy and analgesic efficacy in a murine pain model of tail-flick reflex
General
Type : Sulfur Compound, Urea derivative, Trifluoro, Adamantyl
Chemical_Nomenclature : 1-(1-adamantyl)-3-[4-(trifluoromethoxy)phenyl]sulfonylurea
Canonical SMILES : C1C2CC3CC1CC(C2)(C3)NC(=O)NS(=O)(=O)C4=CC=C(C=C4)OC(F)(F)F
InChI : InChI=1S\/C18H21F3N2O4S\/c19-18(20,21)27-14-1-3-15(4-2-14)28(25,26)23-16(24)22-17-8-11-5-12(9-17)7-13(6-11)10-17\/h1-4,11-13H,5-10H2,(H2,22,23,24)
InChIKey : SNXCHFLIKVETBL-UHFFFAOYSA-N
Other name(s) : 1-(1-Adamantyl)-3-[4-(trifluoromethoxy)phenyl]sulfonylurea || SCHEMBL25290940 || SCHEMBL25808731
MW : 418.4
Formula : C18H21F3N2O4S
CAS_number :
PubChem : 168939804
UniChem : SNXCHFLIKVETBL-UHFFFAOYSA-N
Target
Families : Adamantyl-trifluoromethoxy-phenyl-sulfonylurea-4f ligand of proteins in family
Epoxide_hydrolase
Protein :
human-EPHX2
References (1)
| Title : Evaluation of the Therapeutic Potential of Sulfonyl Urea Derivatives as Soluble Epoxide Hydrolase (sEH) Inhibitors - Kundu_2024_Molecules_29_3036 |
| Author(s) : Kundu B , Dvoracsko S , Basu A , Pommerolle L , Kim KA , Wood CM , Gibbs E , Behee M , Tarasova NI , Cinar R , Iyer MR |
| Ref : Molecules , 29 :3036 , 2024 |
| Abstract : |
| PubMedSearch : Kundu_2024_Molecules_29_3036 |
| PubMedID: 38998987 |