Gamma-carboline-phenothiazine-Cpd7b
NMDAR GluN1-1a/2A IC50 1.7 +/- 0.3 microM; NMDAR GluN1-1a/2B 2.7 +/- 0.3 microM; rhAChE IC50 6.2 +/- 1.0 microM; BChE IC50 0.008 +/- 0.001 microM
General
Type : beta-carboline, Phenothiazine, Indole, NMDA-Ligand, Multitarget
Chemical_Nomenclature : 3-(8-fluoropyrido[4,3-b]indol-5-yl)-1-phenothiazin-10-ylpropan-1-one
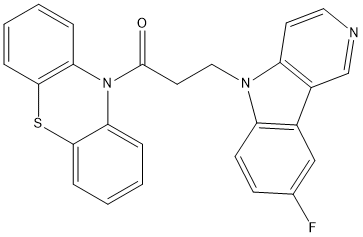
Canonical SMILES : C1=CC=C2C(=C1)N(C3=CC=CC=C3S2)C(=O)CCN4C5=C(C=C(C=C5)F)C6=C4C=CN=C6
InChI : InChI=1S\/C26H18FN3OS\/c27-17-9-10-20-18(15-17)19-16-28-13-11-21(19)29(20)14-12-26(31)30-22-5-1-3-7-24(22)32-25-8-4-2-6-23(25)30\/h1-11,13,15-16H,12,14H2
InChIKey : GUDOKVFOWONHLZ-UHFFFAOYSA-N
Other name(s) : CHEMBL4864593 || BDBM50572067
MW : 439.5
Formula : C26H18FN3OS
CAS_number :
PubChem : 164624952
UniChem : GUDOKVFOWONHLZ-UHFFFAOYSA-N
Target
Families : Gamma-carboline-phenothiazine-Cpd7b ligand of proteins in family
BCHE
Protein :
human-BCHE
References (1)
| Title : Evaluation of gamma-carboline-phenothiazine conjugates as simultaneous NMDA receptor blockers and cholinesterase inhibitors - Schwarthoff_2021_Bioorg.Med.Chem_46_116355 |
| Author(s) : Schwarthoff S , Tischer N , Sager H , Schatz B , Rohrbach MM , Raztsou I , Robaa D , Gaube F , Arndt HD , Winckler T |
| Ref : Bioorganic & Medicinal Chemistry , 46 :116355 , 2021 |
| Abstract : |
| PubMedSearch : Schwarthoff_2021_Bioorg.Med.Chem_46_116355 |
| PubMedID: 34391122 |