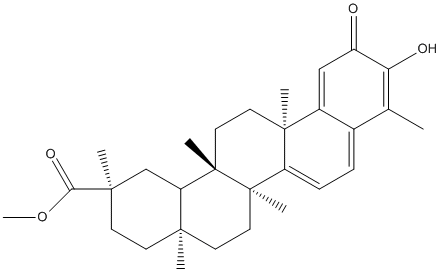
Pristimerin
King et al. found two naturally occurring terpendoids, pristimerin and euphol. Pristimerin (IC50 = 93 +/- 8 nM). Not specific enough for futher development
General
Chemical_Nomenclature : methyl (2R,4aS,6aR,6aS,14aS,14bR)-10-hydroxy-2,4a,6a,6a,9,14a-hexamethyl-11-oxo-1,3,4,5,6,13,14,14b-octahydropicene-2-carboxylate
Canonical SMILES : CC1=C(C(=O)C=C2C1=CC=C3C2(CCC4(C3(CCC5(C4CC(CC5)(C)C(=O)OC)C)C)C)C)O
InChI : InChI=1S\/C30H40O4\/c1-18-19-8-9-22-28(4,20(19)16-21(31)24(18)32)13-15-30(6)23-17-27(3,25(33)34-7)11-10-26(23,2)12-14-29(22,30)5\/h8-9,16,23,32H,10-15,17H2,1-7H3\/t23-,26-,27-,28+,29-,30+\/m1\/s1
InChIKey : JFACETXYABVHFD-WXPPGMDDSA-N
Other name(s) : Celastrol-methylether || GNF-PF-476 || CHEBI:8416 || Pristimerine
MW : 464.63
Formula : C30H40O4
CAS_number : 1258-84-0
PubChem : 159516
UniChem : JFACETXYABVHFD-WXPPGMDDSA-N
Target
Families : Pristimerin ligand of proteins in family
Monoglyceridelipase_lysophospholip
Protein :
human-MGLL
References (18)
| Title : ABHD2 activity is not required for the non-genomic action of progesterone on human sperm - Arnolds_2025_bioRxiv__ |
| Author(s) : Arnolds O , Carter EM , Edwards M , Wigren E , Homan E , Ribera P , Bentley K , Haraldsson M , Theo-Emegano N , Loppnau P , Szewczyk MM , Cao MA , Barsyte-Lovejoy D , Vester K , Thrun A , Amaral A , Lesche R , Munchow J , Zhu WF , Temme L , Brenker C , Strunker T , Sundstrom M , Todd MH , Edwards AM , Tredup C , Gileadi O |
| Ref : bioRxiv , : , 2025 |
| Abstract : |
| PubMedSearch : Arnolds_2025_bioRxiv__ |
| PubMedID: |
| Gene_locus related to this paper: human-ABHD2 |
| Title : ABHD2 activity is not required for the non-genomic action of progesterone on human sperm - Arnolds_2025_bioRxiv__ |
| Author(s) : Arnolds O , Carter EM , Edwards M , Wigren E , Homan E , Ribera P , Bentley K , Haraldsson M , Theo-Emegano N , Loppnau P , Szewczyk MM , Cao MA , Barsyte-Lovejoy D , Vester K , Thrun A , Amaral A , Lesche R , Munchow J , Zhu WF , Temme L , Brenker C , Strunker T , Sundstrom M , Todd MH , Edwards AM , Tredup C , Gileadi O |
| Ref : bioRxiv , : , 2025 |
| Abstract : |
| PubMedSearch : Arnolds_2025_bioRxiv__ |
| PubMedID: |
| Gene_locus related to this paper: human-ABHD2 |
| Title : Correlation Between In Silico Docking\/Simulation Results and In Vitro MAGL Inhibition Potency of Selected Triterpenes - Masocha_2025_Curr.Issues.Mol.Biol_47_ |
| Author(s) : Masocha W , Khedr MA |
| Ref : Curr Issues Mol Biol , 47 : , 2025 |
| Abstract : |
| PubMedSearch : Masocha_2025_Curr.Issues.Mol.Biol_47_ |
| PubMedID: 41020813 |
| Title : ABHD2 activity is not required for the non-genomic action of progesterone on human sperm - Arnolds_2025_bioRxiv__ |
| Author(s) : Arnolds O , Carter EM , Edwards M , Wigren E , Homan E , Ribera P , Bentley K , Haraldsson M , Theo-Emegano N , Loppnau P , Szewczyk MM , Cao MA , Barsyte-Lovejoy D , Vester K , Thrun A , Amaral A , Lesche R , Munchow J , Zhu WF , Temme L , Brenker C , Strunker T , Sundstrom M , Todd MH , Edwards AM , Tredup C , Gileadi O |
| Ref : bioRxiv , : , 2025 |
| Abstract : |
| PubMedSearch : Arnolds_2025_bioRxiv__ |
| PubMedID: |
| Gene_locus related to this paper: human-ABHD2 |
| Title : Correlation Between In Silico Docking\/Simulation Results and In Vitro MAGL Inhibition Potency of Selected Triterpenes - Masocha_2025_Curr.Issues.Mol.Biol_47_ |
| Author(s) : Masocha W , Khedr MA |
| Ref : Curr Issues Mol Biol , 47 : , 2025 |
| Abstract : |
| PubMedSearch : Masocha_2025_Curr.Issues.Mol.Biol_47_ |
| PubMedID: 41020813 |
| Title : Correlation Between In Silico Docking\/Simulation Results and In Vitro MAGL Inhibition Potency of Selected Triterpenes - Masocha_2025_Curr.Issues.Mol.Biol_47_ |
| Author(s) : Masocha W , Khedr MA |
| Ref : Curr Issues Mol Biol , 47 : , 2025 |
| Abstract : |
| PubMedSearch : Masocha_2025_Curr.Issues.Mol.Biol_47_ |
| PubMedID: 41020813 |
| Title : Pristimerin, a triterpene that inhibits monoacylglycerol lipase activity, prevents the development of paclitaxel-induced allodynia in mice - Al-Romaiyan_2022_Front.Pharmacol_13_944502 |
| Author(s) : Al-Romaiyan A , Masocha W |
| Ref : Front Pharmacol , 13 :944502 , 2022 |
| Abstract : |
| PubMedSearch : Al-Romaiyan_2022_Front.Pharmacol_13_944502 |
| PubMedID: 36016571 |
| Title : Pristimerin, a triterpene that inhibits monoacylglycerol lipase activity, prevents the development of paclitaxel-induced allodynia in mice - Al-Romaiyan_2022_Front.Pharmacol_13_944502 |
| Author(s) : Al-Romaiyan A , Masocha W |
| Ref : Front Pharmacol , 13 :944502 , 2022 |
| Abstract : |
| PubMedSearch : Al-Romaiyan_2022_Front.Pharmacol_13_944502 |
| PubMedID: 36016571 |
| Title : Pristimerin, a triterpene that inhibits monoacylglycerol lipase activity, prevents the development of paclitaxel-induced allodynia in mice - Al-Romaiyan_2022_Front.Pharmacol_13_944502 |
| Author(s) : Al-Romaiyan A , Masocha W |
| Ref : Front Pharmacol , 13 :944502 , 2022 |
| Abstract : |
| PubMedSearch : Al-Romaiyan_2022_Front.Pharmacol_13_944502 |
| PubMedID: 36016571 |
| Title : Monoglyceride lipase: Structure and inhibitors - Scalvini_2016_Chem.Phys.Lipids_197_13 |
| Author(s) : Scalvini L , Piomelli D , Mor M |
| Ref : Chemistry & Physic of Lipids , 197 :13 , 2016 |
| Abstract : |
| PubMedSearch : Scalvini_2016_Chem.Phys.Lipids_197_13 |
| PubMedID: 26216043 |
| Title : Monoglyceride lipase: Structure and inhibitors - Scalvini_2016_Chem.Phys.Lipids_197_13 |
| Author(s) : Scalvini L , Piomelli D , Mor M |
| Ref : Chemistry & Physic of Lipids , 197 :13 , 2016 |
| Abstract : |
| PubMedSearch : Scalvini_2016_Chem.Phys.Lipids_197_13 |
| PubMedID: 26216043 |
| Title : Monoglyceride lipase: Structure and inhibitors - Scalvini_2016_Chem.Phys.Lipids_197_13 |
| Author(s) : Scalvini L , Piomelli D , Mor M |
| Ref : Chemistry & Physic of Lipids , 197 :13 , 2016 |
| Abstract : |
| PubMedSearch : Scalvini_2016_Chem.Phys.Lipids_197_13 |
| PubMedID: 26216043 |
| Title : The antinociceptive triterpene beta-amyrin inhibits 2-arachidonoylglycerol (2-AG) hydrolysis without directly targeting cannabinoid receptors - Chicca_2012_Br.J.Pharmacol_167_1596 |
| Author(s) : Chicca A , Marazzi J , Gertsch J |
| Ref : British Journal of Pharmacology , 167 :1596 , 2012 |
| Abstract : |
| PubMedSearch : Chicca_2012_Br.J.Pharmacol_167_1596 |
| PubMedID: 22646533 |
| Title : The antinociceptive triterpene beta-amyrin inhibits 2-arachidonoylglycerol (2-AG) hydrolysis without directly targeting cannabinoid receptors - Chicca_2012_Br.J.Pharmacol_167_1596 |
| Author(s) : Chicca A , Marazzi J , Gertsch J |
| Ref : British Journal of Pharmacology , 167 :1596 , 2012 |
| Abstract : |
| PubMedSearch : Chicca_2012_Br.J.Pharmacol_167_1596 |
| PubMedID: 22646533 |
| Title : The antinociceptive triterpene beta-amyrin inhibits 2-arachidonoylglycerol (2-AG) hydrolysis without directly targeting cannabinoid receptors - Chicca_2012_Br.J.Pharmacol_167_1596 |
| Author(s) : Chicca A , Marazzi J , Gertsch J |
| Ref : British Journal of Pharmacology , 167 :1596 , 2012 |
| Abstract : |
| PubMedSearch : Chicca_2012_Br.J.Pharmacol_167_1596 |
| PubMedID: 22646533 |
| Title : Discovery of potent and reversible monoacylglycerol lipase inhibitors - King_2009_Chem.Biol_16_1045 |
| Author(s) : King AR , Dotsey EY , Lodola A , Jung KM , Ghomian A , Qiu Y , Fu J , Mor M , Piomelli D |
| Ref : Chemical Biology , 16 :1045 , 2009 |
| Abstract : |
| PubMedSearch : King_2009_Chem.Biol_16_1045 |
| PubMedID: 19875078 |
| Title : Discovery of potent and reversible monoacylglycerol lipase inhibitors - King_2009_Chem.Biol_16_1045 |
| Author(s) : King AR , Dotsey EY , Lodola A , Jung KM , Ghomian A , Qiu Y , Fu J , Mor M , Piomelli D |
| Ref : Chemical Biology , 16 :1045 , 2009 |
| Abstract : |
| PubMedSearch : King_2009_Chem.Biol_16_1045 |
| PubMedID: 19875078 |
| Title : Discovery of potent and reversible monoacylglycerol lipase inhibitors - King_2009_Chem.Biol_16_1045 |
| Author(s) : King AR , Dotsey EY , Lodola A , Jung KM , Ghomian A , Qiu Y , Fu J , Mor M , Piomelli D |
| Ref : Chemical Biology , 16 :1045 , 2009 |
| Abstract : |
| PubMedSearch : King_2009_Chem.Biol_16_1045 |
| PubMedID: 19875078 |