Quinazolin-carbamate-2p
the most potent inhibitor of BChE || IC50 hBCHE 13nM highly selective. the most potent inhibitor of BChE
General
Type : Quinazoline, Carbamate, Sulfur Compound, Thiophen
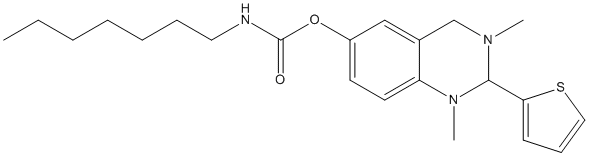
Chemical_Nomenclature : (1,3-dimethyl-2-thiophen-2-yl-2,4-dihydroquinazolin-6-yl) N-heptylcarbamate
Canonical SMILES : N2(CC1=CC(=CC=C1N(C2C3=CC=CS3)C)OC(N(CCCCCCC)[H])=O)C || CCCCCCCNC(=O)OC1=CC2=C(C=C1)N(C(N(C2)C)C3=CC=CS3)C
InChI : InChI=1S\/C22H31N3O2S\/c1-4-5-6-7-8-13-23-22(26)27-18-11-12-19-17(15-18)16-24(2)21(25(19)3)20-10-9-14-28-20\/h9-12,14-15,21H,4-8,13,16H2,1-3H3,(H,23,26)
InChIKey : UWWRFVZRXFROOL-UHFFFAOYSA-N
Other name(s) : 1,3-Dimethyl-2-(thiophen-2-yl)-1,2,3,4-tetrahydroquinazolin-6-yl n-heptylcarbamate || CHEMBL3787613 || BDBM50160078 || Compound 2p
MW : 401.57
Formula : C22H31N3O2S
CAS_number :
PubChem : 127033914
UniChem : UWWRFVZRXFROOL-UHFFFAOYSA-N
Target
Families : Quinazolin-carbamate-2p ligand of proteins in family
BCHE
References (1)
| Title : Discovery of Highly Selective and Nanomolar Carbamate-Based Butyrylcholinesterase Inhibitors by Rational Investigation into Their Inhibition Mode - Sawatzky_2016_J.Med.Chem_59_2067 |
| Author(s) : Sawatzky E , Wehle S , Kling B , Wendrich J , Bringmann G , Sotriffer CA , Heilmann J , Decker M |
| Ref : Journal of Medicinal Chemistry , 59 :2067 , 2016 |
| Abstract : |
| PubMedSearch : Sawatzky_2016_J.Med.Chem_59_2067 |
| PubMedID: 26886849 |