hBChE-IN-1-Cpd4
IC50 tau aggregation 20 microM; IC50 Abeta40 aggregation 4.3 microM; IC50 eeAChE 0.18 microM; IC50 hAChE 7.84 +/- 0.86 microM; IC50 eqBChE 0.088 microM; IC50 hBChE 0.0070 +/- 0.0005 microM
General
Type : Quinolizine, Xanthene, Sulfur Compound, Multitarget, Alkyl linked bis-ligand
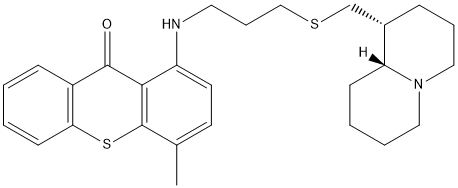
Chemical_Nomenclature : 1-[3-[[(1R,9aR)-2,3,4,6,7,8,9,9a-octahydro-1H-quinolizin-1-yl]methylsulfanyl]propylamino]-4-methylthioxanthen-9-one
Canonical SMILES : CC1=C2C(=C(C=C1)NCCCSCC3CCCN4C3CCCC4)C(=O)C5=CC=CC=C5S2
InChI : InChI=1S\/C27H34N2OS2\/c1-19-12-13-22(25-26(30)21-9-2-3-11-24(21)32-27(19)25)28-14-7-17-31-18-20-8-6-16-29-15-5-4-10-23(20)29\/h2-3,9,11-13,20,23,28H,4-8,10,14-18H2,1H3\/t20-,23+\/m0\/s1
InChIKey : RTSVPQGEMHVGDV-NZQKXSOJSA-N
Other name(s) : hBChE-IN-1 || HY-149273 || CS-0774444
MW : 466.7
Formula : C27H34N2OS2
CAS_number :
PubChem : 168355642
UniChem : RTSVPQGEMHVGDV-NZQKXSOJSA-N
Target
Families : hBChE-IN-1-Cpd4 ligand of proteins in family
BCHE
Protein :
human-BCHE
References (1)
| Title : Thioxanthenone-based derivatives as multitarget therapeutic leads for Alzheimer's disease - Tonelli_2023_Eur.J.Med.Chem_250_115169 |
| Author(s) : Tonelli M , Catto M , Sabate R , Francesconi V , Laurini E , Pricl S , Pisani L , Miniero DV , Liuzzi GM , Gatta E , Relini A , Gavin R , Del Rio JA , Sparatore F , Carotti A |
| Ref : Eur Journal of Medicinal Chemistry , 250 :115169 , 2023 |
| Abstract : |
| PubMedSearch : Tonelli_2023_Eur.J.Med.Chem_250_115169 |
| PubMedID: 36753881 |