6QAB-HUQ
IC50 6.2 nM for human BChE Residual activity of 92% for human AChE. Compound 10 of the paper Meden et al. derived from Tryptophan
General
Type : Derivative of Tryptophan,Indole
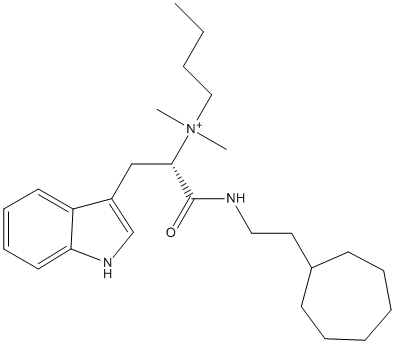
Chemical_Nomenclature : butyl-[(2S)-1-(2-cycloheptylethylamino)-3-(1H-indol-3-yl)-1-oxopropan-2-yl]-dimethylazanium
Canonical SMILES : CCCC[N+](C)(C)C(CC1=CNC2=CC=CC=C21)C(=O)NCCC3CCCCCC3
InChI : InChI=1S\/C26H41N3O\/c1-4-5-18-29(2,3)25(19-22-20-28-24-15-11-10-14-23(22)24)26(30)27-17-16-21-12-8-6-7-9-13-21\/h10-11,14-15,20-21,25,28H,4-9,12-13,16-19H2,1-3H3\/p+1\/t25-\/m0\/s1
InChIKey : KYGLOTKSZWORPK-VWLOTQADSA-O
Other name(s) : HUQ
MW : 412.6
Formula : C26H42N3O+
CAS_number :
PubChem : 138753257
UniChem : KYGLOTKSZWORPK-VWLOTQADSA-O
IUPHAR :
Wikipedia :
Target
Families : 6QAB-HUQ ligand of proteins in family: BCHE
Stucture : 6QAB Human Butyrylcholinesterase in complex with (S)-N-(1-((2-cycloheptylethyl)amino)-3-(1H-indol-3-yl)-1-oxopropan-2-yl)-N,N-dimethylbutan-1-aminium
Protein : human-BCHE
References (1)
| Title : Tryptophan-derived butyrylcholinesterase inhibitors as promising leads against Alzheimer's disease - Meden_2019_Chem.Commun.(Camb)_55_3765 |
| Author(s) : Meden A , Knez D , Jukic M , Brazzolotto X , Grsic M , Pislar A , Zahirovic A , Kos J , Nachon F , Svete J , Gobec S , Groselj U |
| Ref : Chem Commun (Camb) , 55 :3765 , 2019 |
| Abstract : Meden_2019_Chem.Commun.(Camb)_55_3765 |
| ESTHER : Meden_2019_Chem.Commun.(Camb)_55_3765 |
| PubMedSearch : Meden_2019_Chem.Commun.(Camb)_55_3765 |
| PubMedID: 30864579 |
| Gene_locus related to this paper: human-BCHE |