D-tubocurarine
D form isolated from Chondodendron tomentosum (Menispermaceae) the d-form and dimethylether acts as skeletal muscle relaxant Nicotinic. Active ingredient in CURARE. Ligand of small conductance Ca2+-activated K+ (SKca) channels
General
Type : Natural,Alkaloid,Neuromuscular Nondepolarizing Agents,Not A\/B H target,Drug
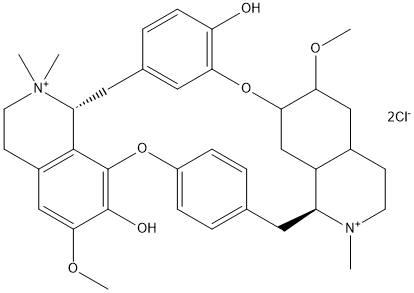
Chemical_Nomenclature : 1',12'-Dihydroxy-6,6'-dimethoxy-2,2'2'-trimethyltubocurarinium chloride
Canonical SMILES : CN1CCC2=CC(=C3C=C2C1CC4=CC=C(C=C4)OC5=C6C(CC7=CC(=C(C=C7)O)O3)[N+](CCC6=CC(=C5O)OC)(C)C)OC
InChI : InChI=1S\/C37H40N2O6\/c1-38-14-12-24-19-32(42-4)33-21-27(24)28(38)16-22-6-9-26(10-7-22)44-37-35-25(20-34(43-5)36(37)41)13-15-39(2,3)29(35)17-23-8-11-30(40)31(18-23)45-33\/h6-11,18-21,28-29H,12-17H2,1-5H3,(H-,40,41)\/p+1\/t28-,29+\/m0\/s1
InChIKey : JFJZZMVDLULRGK-URLMMPGGSA-O
Other name(s) : Tubadil,Delacurarine,Currin-HAF,Tubarine,Intocostrin
MW : 681.70
Formula : C37H42Cl2N2O6
CAS_number : 6989-98-6
PubChem : 6000
UniChem : JFJZZMVDLULRGK-URLMMPGGSA-O
IUPHAR : 2294
Wikipedia : Tubocurarine_chloride
Target
References (11)
| Title : The influence of peripheral site ligands on the reaction of symmetric and chiral organophosphates with wildtype and mutant acetylcholinesterases - Radic_1999_Chem.Biol.Interact_119-120_111 |
| Author(s) : Radic Z , Taylor P |
| Ref : Chemico-Biological Interactions , 119-120 :111 , 1999 |
| Abstract : Radic_1999_Chem.Biol.Interact_119-120_111 |
| ESTHER : Radic_1999_Chem.Biol.Interact_119-120_111 |
| PubMedSearch : Radic_1999_Chem.Biol.Interact_119-120_111 |
| PubMedID: 10421444 |
| Title : Cloning and expression of acetylcholinesterase from Bungarus fasciatus venom. A new type of cooh-terminal domain\; involvement of a positively charged residue in the peripheral site - Cousin_1996_J.Biol.Chem_271_15099 |
| Author(s) : Cousin X , Bon S , Duval N , Massoulie J , Bon C |
| Ref : Journal of Biological Chemistry , 271 :15099 , 1996 |
| Abstract : Cousin_1996_J.Biol.Chem_271_15099 |
| ESTHER : Cousin_1996_J.Biol.Chem_271_15099 |
| PubMedSearch : Cousin_1996_J.Biol.Chem_271_15099 |
| PubMedID: 8662867 |
| Gene_locus related to this paper: bunfa-ACHE |
| Title : Comparative use of muscle relaxants and their reversal in three European countries: a survey in France, Germany and Great Britain - Osmer_1996_Eur.J.Anaesthesiol_13_389 |
| Author(s) : Osmer C , Vogele C , Zickmann B , Hempelmann G |
| Ref : European Journal of Anaesthesiology , 13 :389 , 1996 |
| Abstract : Osmer_1996_Eur.J.Anaesthesiol_13_389 |
| ESTHER : Osmer_1996_Eur.J.Anaesthesiol_13_389 |
| PubMedSearch : Osmer_1996_Eur.J.Anaesthesiol_13_389 |
| PubMedID: 8842663 |
| Title : Edrophonium increases mivacurium concentrations during constant mivacurium infusion, and large doses minimally antagonize paralysis [see comments] - Hart_1995_Anesthesiology_82_912 |
| Author(s) : Hart PS , Wright PM , Brown R , Lau M , Sharma ML , Miller RD , Gruenke L , Fisher DM |
| Ref : Anesthesiology , 82 :912 , 1995 |
| Abstract : Hart_1995_Anesthesiology_82_912 |
| ESTHER : Hart_1995_Anesthesiology_82_912 |
| PubMedSearch : Hart_1995_Anesthesiology_82_912 |
| PubMedID: 7717563 |
| Title : Differential effects of peripheral site ligands on Torpedo and chicken acetylcholinesterase - Eichler_1994_Mol.Pharmacol_45_335 |
| Author(s) : Eichler J , Anselmet A , Sussman JL , Massoulie J , Silman I |
| Ref : Molecular Pharmacology , 45 :335 , 1994 |
| Abstract : Eichler_1994_Mol.Pharmacol_45_335 |
| ESTHER : Eichler_1994_Mol.Pharmacol_45_335 |
| PubMedSearch : Eichler_1994_Mol.Pharmacol_45_335 |
| PubMedID: 8114681 |
| Title : Kinetic analysis of neuromuscular blockade. I. Relationship between twitch depression and stimulation frequency after d-tubocurarine administration - Tajima_1994_Biol.Pharm.Bull_17_1083 |
| Author(s) : Tajima T , Kaneko K , Hatanaka T , Aiba T , Katayama K , Koizumi T |
| Ref : Biol Pharm Bull , 17 :1083 , 1994 |
| Abstract : Tajima_1994_Biol.Pharm.Bull_17_1083 |
| ESTHER : Tajima_1994_Biol.Pharm.Bull_17_1083 |
| PubMedSearch : Tajima_1994_Biol.Pharm.Bull_17_1083 |
| PubMedID: 7820113 |
| Title : Binding of 125I-fasciculin to rat brain acetylcholinesterase. The complex still binds diisopropyl fluorophosphate - Marchot_1993_J.Biol.Chem_268_12458 |
| Author(s) : Marchot P , Khelif A , Ji YH , Mansuelle P , Bougis PE |
| Ref : Journal of Biological Chemistry , 268 :12458 , 1993 |
| Abstract : Marchot_1993_J.Biol.Chem_268_12458 |
| ESTHER : Marchot_1993_J.Biol.Chem_268_12458 |
| PubMedSearch : Marchot_1993_J.Biol.Chem_268_12458 |
| PubMedID: 8509385 |
| Title : Non-quantal release of acetylcholine affects polyneuronal innervation on developing rat muscle fibres - Vyskocil_1993_Eur.J.Neurosci_5_1677 |
| Author(s) : Vyskocil F , Vrbova G |
| Ref : European Journal of Neuroscience , 5 :1677 , 1993 |
| Abstract : Vyskocil_1993_Eur.J.Neurosci_5_1677 |
| ESTHER : Vyskocil_1993_Eur.J.Neurosci_5_1677 |
| PubMedSearch : Vyskocil_1993_Eur.J.Neurosci_5_1677 |
| PubMedID: 8124519 |
| Title : Structure-activity relationship of reversible cholinesterase inhibitors including paraquat - Seto_1988_Arch.Toxicol_62_37 |
| Author(s) : Seto Y , Shinohara T |
| Ref : Archives of Toxicology , 62 :37 , 1988 |
| Abstract : Seto_1988_Arch.Toxicol_62_37 |
| ESTHER : Seto_1988_Arch.Toxicol_62_37 |
| PubMedSearch : Seto_1988_Arch.Toxicol_62_37 |
| PubMedID: 3190453 |
| Title : Multiple binding of D-tubocurarine to acetylcholinesterase - Zorko_1986_Biochem.Pharmacol_35_2287 |
| Author(s) : Zorko M , Pavlic MR |
| Ref : Biochemical Pharmacology , 35 :2287 , 1986 |
| Abstract : Zorko_1986_Biochem.Pharmacol_35_2287 |
| ESTHER : Zorko_1986_Biochem.Pharmacol_35_2287 |
| PubMedSearch : Zorko_1986_Biochem.Pharmacol_35_2287 |
| PubMedID: 3729986 |
| Title : Interaction of fluorescence probes with acetylcholinesterase. The site and specificity of propidium binding - Taylor_1975_Biochemistry_14_1989 |
| Author(s) : Taylor P , Lappi S |
| Ref : Biochemistry , 14 :1989 , 1975 |
| Abstract : Taylor_1975_Biochemistry_14_1989 |
| ESTHER : Taylor_1975_Biochemistry_14_1989 |
| PubMedSearch : Taylor_1975_Biochemistry_14_1989 |
| PubMedID: 1125207 |