DO34
General
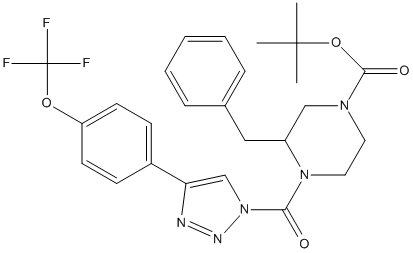
Type : Trifluoro,Piperazine,Carbamate,tert-Butyloxycarbonyl,tert-Butyl
Chemical_Nomenclature : tert-butyl 3-benzyl-4-[4-[4-(trifluoromethoxy)phenyl]triazole-1-carbonyl]piperazine-1-carboxylate
Canonical SMILES : CC(C)(C)OC(=O)N1CCN(C(C1)CC2=CC=CC=C2)C(=O)N3C=C(N=N3)C4=CC=C(C=C4)OC(F)(F)F
InChI : InChI=1S\/C26H28F3N5O4\/c1-25(2,3)38-24(36)32-13-14-33(20(16-32)15-18-7-5-4-6-8-18)23(35)34-17-22(30-31-34)19-9-11-21(12-10-19)37-26(27,28)29\/h4-12,17,20H,13-16H2,1-3H3
InChIKey : CQGMWUWJVBKTRM-UHFFFAOYSA-N
Other name(s) : SCHEMBL18921287,DO-34,J3.619.706D,3-(Phenylmethyl)-4-[[4-[4-(trifluoromethoxy)phenyl]-1H-1,2,3-triazol-1-yl]carbonyl]-1-piperazinecarboxylic acid 1,1-dimethylethyl ester
MW : 531.53
Formula : C26H28F3N5O4
CAS_number : 1848233-58-8
PubChem : 129188708
UniChem : CQGMWUWJVBKTRM-UHFFFAOYSA-N
IUPHAR :
Wikipedia :
Target
Families : DO34 ligand of proteins in family: Lipase_3
Stucture :
Protein : human-DAGLA || human-DAGLB
References (5)
| Title : Pharmacological diacylglycerol lipase inhibition impairs contextual fear extinction in mice - Ramos-Medina_2024_Psychopharmacology.(Berl)__ |
| Author(s) : Ramos-Medina L , Rosas-Vidal LE , Patel S |
| Ref : Psychopharmacology (Berl) , : , 2024 |
| Abstract : Ramos-Medina_2024_Psychopharmacology.(Berl)__ |
| ESTHER : Ramos-Medina_2024_Psychopharmacology.(Berl)__ |
| PubMedSearch : Ramos-Medina_2024_Psychopharmacology.(Berl)__ |
| PubMedID: 38182791 |
| Gene_locus related to this paper: human-DAGLA , human-DAGLB |
| Title : Triazole Ureas Act as Diacylglycerol Lipase Inhibitors and Prevent Fasting-Induced Refeeding - Deng_2017_J.Med.Chem_60_428 |
| Author(s) : Deng H , Kooijman S , van den Nieuwendijk AM , Ogasawara D , van der Wel T , van Dalen F , Baggelaar MP , Janssen FJ , van den Berg RJ , den Dulk H , Cravatt BF , Overkleeft HS , Rensen PC , van der Stelt M |
| Ref : Journal of Medicinal Chemistry , 60 :428 , 2017 |
| Abstract : Deng_2017_J.Med.Chem_60_428 |
| ESTHER : Deng_2017_J.Med.Chem_60_428 |
| PubMedSearch : Deng_2017_J.Med.Chem_60_428 |
| PubMedID: 27992221 |
| Title : Investigation of Diacylglycerol Lipase Alpha Inhibition in the Mouse Lipopolysaccharide Inflammatory Pain Model - Wilkerson_2017_J.Pharmacol.Exp.Ther_363_394 |
| Author(s) : Wilkerson JL , Donvito G , Grim TW , Abdullah RA , Ogasawara D , Cravatt BF , Lichtman AH |
| Ref : Journal of Pharmacology & Experimental Therapeutics , 363 :394 , 2017 |
| Abstract : Wilkerson_2017_J.Pharmacol.Exp.Ther_363_394 |
| ESTHER : Wilkerson_2017_J.Pharmacol.Exp.Ther_363_394 |
| PubMedSearch : Wilkerson_2017_J.Pharmacol.Exp.Ther_363_394 |
| PubMedID: 28970359 |
| Gene_locus related to this paper: mouse-q6wqj1 |
| Title : Rapid and profound rewiring of brain lipid signaling networks by acute diacylglycerol lipase inhibition - Ogasawara_2016_Proc.Natl.Acad.Sci.U.S.A_113_26 |
| Author(s) : Ogasawara D , Deng H , Viader A , Baggelaar MP , Breman A , den Dulk H , van den Nieuwendijk AM , Soethoudt M , van der Wel T , Zhou J , Overkleeft HS , Sanchez-Alavez M , Mori S , Nguyen W , Conti B , Liu X , Chen Y , Liu QS , Cravatt BF , van der Stelt M |
| Ref : Proc Natl Acad Sci U S A , 113 :26 , 2016 |
| Abstract : Ogasawara_2016_Proc.Natl.Acad.Sci.U.S.A_113_26 |
| ESTHER : Ogasawara_2016_Proc.Natl.Acad.Sci.U.S.A_113_26 |
| PubMedSearch : Ogasawara_2016_Proc.Natl.Acad.Sci.U.S.A_113_26 |
| PubMedID: 26668358 |
| Gene_locus related to this paper: human-DAGLA , human-DAGLB |
| Title : Coordinated regulation of endocannabinoid-mediated retrograde synaptic suppression in the cerebellum by neuronal and astrocytic monoacylglycerol lipase - Liu_2016_Sci.Rep_6_35829 |
| Author(s) : Liu X , Chen Y , Vickstrom CR , Li Y , Viader A , Cravatt BF , Liu QS |
| Ref : Sci Rep , 6 :35829 , 2016 |
| Abstract : Liu_2016_Sci.Rep_6_35829 |
| ESTHER : Liu_2016_Sci.Rep_6_35829 |
| PubMedSearch : Liu_2016_Sci.Rep_6_35829 |
| PubMedID: 27775008 |