Hopeahainol-A
Oligostilbenoid resveratrol derived acetylcholinesterase inhibitor isolated from Hopea hainanensis with an IC50 value of 4.33 microM
General
Type : Polyphenol,Natural,Benzofuran
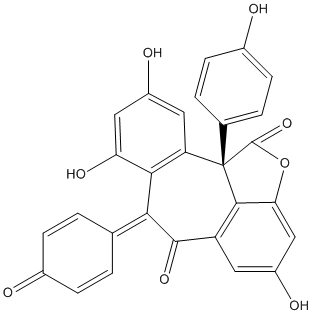
Chemical_Nomenclature : (11bS)-4,8,10-Trihydroxy-7-(4-oxo-2,5-cyclohexadiene-1-ylidene)-11b-(4-hydroxyphenyl)-1,6,7,11b-tetrahydrobenzo[6,7]cyclohepta[1,2,3-cd]benzofuran-1,6-dione
Canonical SMILES : C1=CC(=CC=C1C2=C3C(=CC(=O)C=C3O)C4(C5=C(C=C(C=C5C2=O)O)OC4=O)C6=CC=C(C=C6)O)O
InChI : InChI=1S\/C28H16O8\/c29-15-5-1-13(2-6-15)23-24-20(10-18(32)11-21(24)33)28(14-3-7-16(30)8-4-14)25-19(26(23)34)9-17(31)12-22(25)36-27(28)35\/h1-12,29-31,33H\/t28-\/m0\/s1
InChIKey : ZDIDNYXNHOCPFJ-NDEPHWFRSA-N
Other name(s) : HopA
MW : 480.42
Formula : C28H16O8
CAS_number :
PubChem : 24773316
UniChem : ZDIDNYXNHOCPFJ-NDEPHWFRSA-N
IUPHAR :
Wikipedia :
Target
References (9)
| Title : Hopeahainol A binds reversibly at the acetylcholinesterase (AChE) peripheral site and inhibits enzyme activity with a novel higher order concentration dependence - Rosenberry_2016_Chem.Biol.Interact_259_78 |
| Author(s) : Rosenberry TL , Martin PK , Nix AJ , Wildman SA , Cheung J , Snyder SA , Tan RX |
| Ref : Chemico-Biological Interactions , 259 :78 , 2016 |
| Abstract : Rosenberry_2016_Chem.Biol.Interact_259_78 |
| ESTHER : Rosenberry_2016_Chem.Biol.Interact_259_78 |
| PubMedSearch : Rosenberry_2016_Chem.Biol.Interact_259_78 |
| PubMedID: 27297626 |
| Title : Oligostilbenoids with Acetylcholinesterase Inhibitory Activity from Dipterocarpus alatus - Chen_2014_Planta.Med_80_1641 |
| Author(s) : Chen CJ , Jiang R , Wang G , Jiao RH , Tancharoen C , Sudto K , Vajarothai S , Hannongbua S , Ge HM , Tan RX |
| Ref : Planta Med , 80 :1641 , 2014 |
| Abstract : Chen_2014_Planta.Med_80_1641 |
| ESTHER : Chen_2014_Planta.Med_80_1641 |
| PubMedSearch : Chen_2014_Planta.Med_80_1641 |
| PubMedID: 25317771 |
| Title : Hopeahainol A attenuates memory deficits by targeting beta-amyloid in APP\/PS1 transgenic mice - Zhu_2013_Aging.Cell_12_85 |
| Author(s) : Zhu X , Ye L , Ge H , Chen L , Jiang N , Qian L , Li L , Liu R , Ji S , Zhang S , Jin J , Guan D , Fang W , Tan R , Xu Y |
| Ref : Aging Cell , 12 :85 , 2013 |
| Abstract : Zhu_2013_Aging.Cell_12_85 |
| ESTHER : Zhu_2013_Aging.Cell_12_85 |
| PubMedSearch : Zhu_2013_Aging.Cell_12_85 |
| PubMedID: 23107435 |
| Title : Polyphenolic acetylcholinesterase inhibitors from Hopea chinensis - Yan_2012_Planta.Med_78_1015 |
| Author(s) : Yan T , Wang T , Wei W , Jiang N , Qin YH , Tan RX , Ge HM |
| Ref : Planta Med , 78 :1015 , 2012 |
| Abstract : Yan_2012_Planta.Med_78_1015 |
| ESTHER : Yan_2012_Planta.Med_78_1015 |
| PubMedSearch : Yan_2012_Planta.Med_78_1015 |
| PubMedID: 22628156 |
| Title : Total syntheses of hopeanol and hopeahainol A empowered by a chiral Bronsted acid induced pinacol rearrangement - |
| Author(s) : Snyder SA , Thomas SB , Mayer AC , Breazzano SP |
| Ref : Angew Chem Int Ed Engl , 51 :4080 , 2012 |
| PubMedID: 22431237 |
| Title : Total syntheses of heimiol A, hopeahainol D, and constrained analogues - |
| Author(s) : Snyder SA , Wright NE , Pflueger JJ , Breazzano SP |
| Ref : Angew Chem Int Ed Engl , 50 :8629 , 2011 |
| PubMedID: 21793142 |
| Title : Total synthesis and biological evaluation of the resveratrol-derived polyphenol natural products hopeanol and hopeahainol A - Nicolaou_2010_J.Am.Chem.Soc_132_7540 |
| Author(s) : Nicolaou KC , Kang Q , Wu TR , Lim CS , Chen DY |
| Ref : Journal of the American Chemical Society , 132 :7540 , 2010 |
| Abstract : Nicolaou_2010_J.Am.Chem.Soc_132_7540 |
| ESTHER : Nicolaou_2010_J.Am.Chem.Soc_132_7540 |
| PubMedSearch : Nicolaou_2010_J.Am.Chem.Soc_132_7540 |
| PubMedID: 20462209 |
| Title : Protective effect of hopeahainol A, a novel acetylcholinesterase inhibitor, on hydrogen peroxide-induced injury in PC12 cells - Shi_2009_Environ.Toxicol.Pharmacol_28_30 |
| Author(s) : Shi da H , Wu JH , Ge HM , Tan RX |
| Ref : Environ Toxicol Pharmacol , 28 :30 , 2009 |
| Abstract : Shi_2009_Environ.Toxicol.Pharmacol_28_30 |
| ESTHER : Shi_2009_Environ.Toxicol.Pharmacol_28_30 |
| PubMedSearch : Shi_2009_Environ.Toxicol.Pharmacol_28_30 |
| PubMedID: 21783979 |
| Title : Hopeahainol A: an acetylcholinesterase inhibitor from Hopea hainanensis - Ge_2008_Chemistry_14_376 |
| Author(s) : Ge HM , Zhu CH , Shi da H , Zhang LD , Xie DQ , Yang J , Ng SW , Tan RX |
| Ref : Chemistry , 14 :376 , 2008 |
| Abstract : Ge_2008_Chemistry_14_376 |
| ESTHER : Ge_2008_Chemistry_14_376 |
| PubMedSearch : Ge_2008_Chemistry_14_376 |
| PubMedID: 17943703 |