M9N
General
Type : Pyrimidine,Sulfur Compound
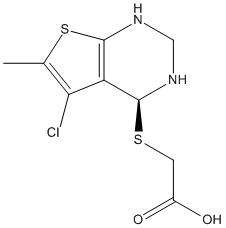
Chemical_Nomenclature : 2-[[(4~{S})-5-chloranyl-6-methyl-1,2,3,4-tetrahydrothieno[2,3-d]pyrimidin-4-yl]sulfanyl]ethanoic acid
Canonical SMILES : Cc1sc2NCN[C@@H](SCC(O)=O)c2c1Cl
InChI : InChI=1S\/C9H11ClN2O2S2\/c1-4-7(10)6-8(15-2-5(13)14)11-3-12-9(6)16-4\/h8,11-12H,2-3H2,1H3,(H,13,14)\/t8-\/m0\/s1
InChIKey : YXTHLXJCVDULSY-QMMMGPOBSA-N
Other name(s) :
MW : 278.78
Formula : C9H11ClN2O2S2
CAS_number :
PubChem : 145946081
UniChem : YXTHLXJCVDULSY-QMMMGPOBSA-N
IUPHAR :
Wikipedia :
Target
Families : M9N ligand of proteins in family: Pectinacetylesterase-Notum
Stucture : 6T2H Furano[2,3-d]prymidine amides as human Notum inhibitors 1 (M9N)
Protein : human-NOTUM
References (2)
| Title : Scaffold-hopping identifies furano[2,3-d]pyrimidine amides as potent Notum inhibitors - Atkinson_2019_Bioorg.Med.Chem.Lett__126751 |
| Author(s) : Atkinson BN , Steadman D , Mahy W , Zhao Y , Sipthorp J , Bayle ED , Svensson F , Papageorgiou G , Jeganathan F , Frew S , Monaghan A , Bictash M , Jones EY , Fish PV |
| Ref : Bioorganic & Medicinal Chemistry Lett , :126751 , 2019 |
| Abstract : Atkinson_2019_Bioorg.Med.Chem.Lett__126751 |
| ESTHER : Atkinson_2019_Bioorg.Med.Chem.Lett__126751 |
| PubMedSearch : Atkinson_2019_Bioorg.Med.Chem.Lett__126751 |
| PubMedID: 31862412 |
| Gene_locus related to this paper: human-NOTUM |
| Title : Stimulation of cortical bone formation with thienopyrimidine based inhibitors of Notum Pectinacetylesterase - Tarver_2016_Bioorg.Med.Chem.Lett_26_1525 |
| Author(s) : Tarver JE, Jr. , Pabba PK , Barbosa J , Han Q , Gardyan MW , Brommage R , Thompson AY , Schmidt JM , Wilson AG , He W , Lombardo VK , Carson KG , Wilson AGE |
| Ref : Bioorganic & Medicinal Chemistry Lett , 26 :1525 , 2016 |
| Abstract : Tarver_2016_Bioorg.Med.Chem.Lett_26_1525 |
| ESTHER : Tarver_2016_Bioorg.Med.Chem.Lett_26_1525 |
| PubMedSearch : Tarver_2016_Bioorg.Med.Chem.Lett_26_1525 |
| PubMedID: 26897593 |
| Gene_locus related to this paper: human-NOTUM |