K206
General
Type : Oxime, Bispyridinium, Carboxamide
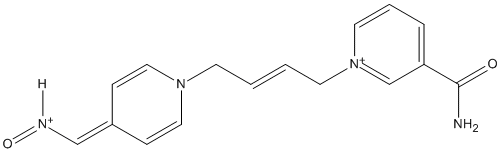
Chemical_Nomenclature : 1-[(E)-4-[4-(nitrosomethylidene)pyridin-1-yl]but-2-enyl]pyridin-1-ium-3-carboxamide
Canonical SMILES : C1=CC(=C[N+](=C1)CC=CCN2C=CC(=CN=O)C=C2)C(=O)N
InChI : InChI=1S\/C16H16N4O2\/c17-16(21)15-4-3-9-20(13-15)8-2-1-7-19-10-5-14(6-11-19)12-18-22\/h1-6,9-13H,7-8H2,(H-,17,21)\/p+1\/b2-1+
InChIKey : LEUXHRLHVOLUNP-OWOJBTEDSA-O
Other name(s) : ZINC59151580
Target
Structure : No structure
Families : No family
References (6)
| Title : Slight difference in the isomeric oximes K206 and K203 makes huge difference for the reactivation of organophosphorus-inhibited AChE: Theoretical and experimental aspects - Polisel_2019_Chem.Biol.Interact_13ChEPon_309_108671 |
| Author(s) : Polisel DA , de Castro AA , Mancini DT , da Cunha EFF , Franca TCC , Ramalho TC , Kuca K |
| Ref : Chemico-Biological Interactions , 309 :108671 , 2019 |
| Abstract : |
| PubMedSearch : Polisel_2019_Chem.Biol.Interact_13ChEPon_309_108671 |
| PubMedID: 31207225 |
| Title : Slight difference in the isomeric oximes K206 and K203 makes huge difference for the reactivation of organophosphorus-inhibited AChE: Theoretical and experimental aspects - Polisel_2019_Chem.Biol.Interact_13ChEPon_309_108671 |
| Author(s) : Polisel DA , de Castro AA , Mancini DT , da Cunha EFF , Franca TCC , Ramalho TC , Kuca K |
| Ref : Chemico-Biological Interactions , 309 :108671 , 2019 |
| Abstract : |
| PubMedSearch : Polisel_2019_Chem.Biol.Interact_13ChEPon_309_108671 |
| PubMedID: 31207225 |
| Title : Slight difference in the isomeric oximes K206 and K203 makes huge difference for the reactivation of organophosphorus-inhibited AChE: Theoretical and experimental aspects - Polisel_2019_Chem.Biol.Interact_13ChEPon_309_108671 |
| Author(s) : Polisel DA , de Castro AA , Mancini DT , da Cunha EFF , Franca TCC , Ramalho TC , Kuca K |
| Ref : Chemico-Biological Interactions , 309 :108671 , 2019 |
| Abstract : |
| PubMedSearch : Polisel_2019_Chem.Biol.Interact_13ChEPon_309_108671 |
| PubMedID: 31207225 |
| Title : Synthesis of monooxime-monocarbamoyl bispyridinium compounds bearing (E)-but-2-ene linker and evaluation of their reactivation activity against tabun- and paraoxon-inhibited acetylcholinesterase - Musilek_2008_J.Enzyme.Inhib.Med.Chem_23_70 |
| Author(s) : Musilek K , Holas O , Kuca K , Jun D , Dohnal V , Opletalova V , Dolezal M |
| Ref : J Enzyme Inhib Med Chem , 23 :70 , 2008 |
| Abstract : |
| PubMedSearch : Musilek_2008_J.Enzyme.Inhib.Med.Chem_23_70 |
| PubMedID: 18341256 |
| Title : Synthesis of monooxime-monocarbamoyl bispyridinium compounds bearing (E)-but-2-ene linker and evaluation of their reactivation activity against tabun- and paraoxon-inhibited acetylcholinesterase - Musilek_2008_J.Enzyme.Inhib.Med.Chem_23_70 |
| Author(s) : Musilek K , Holas O , Kuca K , Jun D , Dohnal V , Opletalova V , Dolezal M |
| Ref : J Enzyme Inhib Med Chem , 23 :70 , 2008 |
| Abstract : |
| PubMedSearch : Musilek_2008_J.Enzyme.Inhib.Med.Chem_23_70 |
| PubMedID: 18341256 |
| Title : Synthesis of monooxime-monocarbamoyl bispyridinium compounds bearing (E)-but-2-ene linker and evaluation of their reactivation activity against tabun- and paraoxon-inhibited acetylcholinesterase - Musilek_2008_J.Enzyme.Inhib.Med.Chem_23_70 |
| Author(s) : Musilek K , Holas O , Kuca K , Jun D , Dohnal V , Opletalova V , Dolezal M |
| Ref : J Enzyme Inhib Med Chem , 23 :70 , 2008 |
| Abstract : |
| PubMedSearch : Musilek_2008_J.Enzyme.Inhib.Med.Chem_23_70 |
| PubMedID: 18341256 |