K456
General
Type : Oxime, Bispyridinium
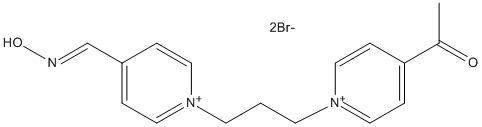
Chemical_Nomenclature : 1-[1-[3-[4-[(E)-hydroxyiminomethyl]pyridin-1-ium-1-yl]propyl]pyridin-1-ium-4-yl]ethanone\;dibromide
Canonical SMILES : C1(=CC=[N+](C=C1)CCC[N+]2=CC=C(C=C2)C(=O)C)C=NO.[Br-].[Br-] || CC(=O)C1=CC=[N+](C=C1)CCC[N+]2=CC=C(C=C2)C=NO.[Br-].[Br-]
InChI : InChI=1S\/C16H18N3O2.2BrH\/c1-14(20)16-5-11-19(12-6-16)8-2-7-18-9-3-15(4-10-18)13-17-21\;\;\/h3-6,9-13H,2,7-8H2,1H3\;2*1H\/q+1\;\;\/p-1
InChIKey : JAIRLQKRIPOMHU-UHFFFAOYSA-M
Other name(s) : K-456
MW : 445.15
Formula : C16H19Br2N3O2
CAS_number :
PubChem : 136030722
UniChem : JAIRLQKRIPOMHU-UHFFFAOYSA-M
Target
Structure : No structure
Families : No family
References (9)
| Title : Reactivation potency of two novel oximes (K456 and K733) against paraoxon-inhibited acetyl and butyrylcholinesterase: In silico and in vitro models - Iqbal_2019_Chem.Biol.Interact_13ChEPon_310_108735 |
| Author(s) : Iqbal A , Malik S , Nurulain SM , Musilek K , Kuca K , Kalasz H , Fatmi MQ |
| Ref : Chemico-Biological Interactions , 310 :108735 , 2019 |
| Abstract : |
| PubMedSearch : Iqbal_2019_Chem.Biol.Interact_13ChEPon_310_108735 |
| PubMedID: 31276662 |
| Title : Reactivation potency of two novel oximes (K456 and K733) against paraoxon-inhibited acetyl and butyrylcholinesterase: In silico and in vitro models - Iqbal_2019_Chem.Biol.Interact_13ChEPon_310_108735 |
| Author(s) : Iqbal A , Malik S , Nurulain SM , Musilek K , Kuca K , Kalasz H , Fatmi MQ |
| Ref : Chemico-Biological Interactions , 310 :108735 , 2019 |
| Abstract : |
| PubMedSearch : Iqbal_2019_Chem.Biol.Interact_13ChEPon_310_108735 |
| PubMedID: 31276662 |
| Title : Reactivation potency of two novel oximes (K456 and K733) against paraoxon-inhibited acetyl and butyrylcholinesterase: In silico and in vitro models - Iqbal_2019_Chem.Biol.Interact_13ChEPon_310_108735 |
| Author(s) : Iqbal A , Malik S , Nurulain SM , Musilek K , Kuca K , Kalasz H , Fatmi MQ |
| Ref : Chemico-Biological Interactions , 310 :108735 , 2019 |
| Abstract : |
| PubMedSearch : Iqbal_2019_Chem.Biol.Interact_13ChEPon_310_108735 |
| PubMedID: 31276662 |
| Title : The evaluation of the reactivating and therapeutic efficacy of three novel bispyridinium oximes (K454, K456, K458) in comparison with the oxime K203 and trimedoxime in tabun-poisoned rats and mice - Kassa_2013_Toxicol.Mech.Methods_23_94 |
| Author(s) : Kassa J , Sepsova V , Musilek K , Horova A |
| Ref : Toxicol Mech Methods , 23 :94 , 2013 |
| Abstract : |
| PubMedSearch : Kassa_2013_Toxicol.Mech.Methods_23_94 |
| PubMedID: 22901042 |
| Title : The evaluation of the reactivating and therapeutic efficacy of three novel bispyridinium oximes (K454, K456, K458) in comparison with the oxime K203 and trimedoxime in tabun-poisoned rats and mice - Kassa_2013_Toxicol.Mech.Methods_23_94 |
| Author(s) : Kassa J , Sepsova V , Musilek K , Horova A |
| Ref : Toxicol Mech Methods , 23 :94 , 2013 |
| Abstract : |
| PubMedSearch : Kassa_2013_Toxicol.Mech.Methods_23_94 |
| PubMedID: 22901042 |
| Title : The evaluation of the reactivating and therapeutic efficacy of three novel bispyridinium oximes (K454, K456, K458) in comparison with the oxime K203 and trimedoxime in tabun-poisoned rats and mice - Kassa_2013_Toxicol.Mech.Methods_23_94 |
| Author(s) : Kassa J , Sepsova V , Musilek K , Horova A |
| Ref : Toxicol Mech Methods , 23 :94 , 2013 |
| Abstract : |
| PubMedSearch : Kassa_2013_Toxicol.Mech.Methods_23_94 |
| PubMedID: 22901042 |
| Title : Mono-oxime bisquaternary acetylcholinesterase reactivators with prop-1,3-diyl linkage-Preparation, in vitro screening and molecular docking - Musilek_2011_Bioorg.Med.Chem_19_754 |
| Author(s) : Musilek K , Komloova M , Holas O , Horova A , Pohanka M , Gunn-Moore F , Dohnal V , Dolezal M , Kuca K |
| Ref : Bioorganic & Medicinal Chemistry , 19 :754 , 2011 |
| Abstract : |
| PubMedSearch : Musilek_2011_Bioorg.Med.Chem_19_754 |
| PubMedID: 21215642 |
| Title : Mono-oxime bisquaternary acetylcholinesterase reactivators with prop-1,3-diyl linkage-Preparation, in vitro screening and molecular docking - Musilek_2011_Bioorg.Med.Chem_19_754 |
| Author(s) : Musilek K , Komloova M , Holas O , Horova A , Pohanka M , Gunn-Moore F , Dohnal V , Dolezal M , Kuca K |
| Ref : Bioorganic & Medicinal Chemistry , 19 :754 , 2011 |
| Abstract : |
| PubMedSearch : Musilek_2011_Bioorg.Med.Chem_19_754 |
| PubMedID: 21215642 |
| Title : Mono-oxime bisquaternary acetylcholinesterase reactivators with prop-1,3-diyl linkage-Preparation, in vitro screening and molecular docking - Musilek_2011_Bioorg.Med.Chem_19_754 |
| Author(s) : Musilek K , Komloova M , Holas O , Horova A , Pohanka M , Gunn-Moore F , Dohnal V , Dolezal M , Kuca K |
| Ref : Bioorganic & Medicinal Chemistry , 19 :754 , 2011 |
| Abstract : |
| PubMedSearch : Musilek_2011_Bioorg.Med.Chem_19_754 |
| PubMedID: 21215642 |