Epicatechin-gallate
General
Type : Derivative of Gallate || Natural || Polyphenol || Benzoate
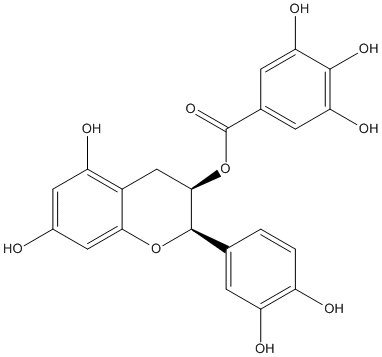
Chemical_Nomenclature : [(2R,3R)-2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-3,4-dihydro-2H-chromen-3-yl] 3,4,5-trihydroxybenzoate
Canonical SMILES : C1C(C(OC2=CC(=CC(=C21)O)O)C3=CC(=C(C=C3)O)O)OC(=O)C4=CC(=C(C(=C4)O)O)O
InChI : InChI=1S\/C22H18O10\/c23-11-6-14(25)12-8-19(32-22(30)10-4-16(27)20(29)17(28)5-10)21(31-18(12)7-11)9-1-2-13(24)15(26)3-9\/h1-7,19,21,23-29H,8H2\/t19-,21-\/m1\/s1
InChIKey : LSHVYAFMTMFKBA-TZIWHRDSSA-N
Other name(s) : (-)-Epicatechin gallate, Epicatechin gallate, (-)-Epicatechin-3-O-gallate, (-)-epicatechingallate, ECG, CHEMBL36327, CHEBI:70255, SCHEMBL39047, ZINC3978503
Target
Families : AlphaBeta_hydrolase, Plant_carboxylesterase
References (8)
| Title : Degradation of epigallocatechin and epicatechin gallates by a novel tannase Tan(Hcw) from Herbaspirillum camelliae - Lei_2021_Microb.Cell.Fact_20_197 |
| Author(s) : Lei J , Zhang Y , Ni X , Yu X , Wang X |
| Ref : Microb Cell Fact , 20 :197 , 2021 |
| Abstract : Lei_2021_Microb.Cell.Fact_20_197 |
| ESTHER : Lei_2021_Microb.Cell.Fact_20_197 |
| PubMedSearch : Lei_2021_Microb.Cell.Fact_20_197 |
| PubMedID: 34641872 |
| Gene_locus related to this paper: 9burk-TanHcw |
| Title : Epicatechin gallate and epigallocatechin gallate are potent inhibitors of human arylacetamide deacetylase - Yasuda_2021_Drug.Metab.Pharmacokinet_39_100397 |
| Author(s) : Yasuda K , Watanabe K , Fukami T , Nakashima S , Ikushiro SI , Nakajima M , Sakaki T |
| Ref : Drug Metab Pharmacokinet , 39 :100397 , 2021 |
| Abstract : Yasuda_2021_Drug.Metab.Pharmacokinet_39_100397 |
| ESTHER : Yasuda_2021_Drug.Metab.Pharmacokinet_39_100397 |
| PubMedSearch : Yasuda_2021_Drug.Metab.Pharmacokinet_39_100397 |
| PubMedID: 34171773 |
| Title : Discovery and characterization of tannase genes in plants: roles in hydrolysis of tannins - Dai_2020_New.Phytol_226_1104 |
| Author(s) : Dai X , Liu Y , Zhuang J , Yao S , Liu L , Jiang X , Zhou K , Wang Y , Xie D , Bennetzen JL , Gao L , Xia T |
| Ref : New Phytol , 226 :1104 , 2020 |
| Abstract : Dai_2020_New.Phytol_226_1104 |
| ESTHER : Dai_2020_New.Phytol_226_1104 |
| PubMedSearch : Dai_2020_New.Phytol_226_1104 |
| PubMedID: 32061142 |
| Gene_locus related to this paper: camsi-a0a5b9g8c2 , jugre-JrTA , fraan-FaTA , vitvi-VvTA , citcl-CcTA , dioka-DkTA , camsi-CsTA |
| Title : (-)-Epicatechin derivate from Orostachys japonicus as potential inhibitor of the human butyrylcholinesterase - Kim_2016_Int.J.Biol.Macromol_91_1033 |
| Author(s) : Kim JH , Lee SH , Lee HW , Sun YN , Jang WH , Yang SY , Jang HD , Kim YH |
| Ref : Int J Biol Macromol , 91 :1033 , 2016 |
| Abstract : Kim_2016_Int.J.Biol.Macromol_91_1033 |
| ESTHER : Kim_2016_Int.J.Biol.Macromol_91_1033 |
| PubMedSearch : Kim_2016_Int.J.Biol.Macromol_91_1033 |
| PubMedID: 27341781 |
| Title : Chemical and biological insights on Cotoneaster integerrimus: A new (-)- epicatechin source for food and medicinal applications - Uysal_2016_Phytomedicine_23_979 |
| Author(s) : Uysal A , Zengin G , Mollica A , Gunes E , Locatelli M , Yilmaz T , Aktumsek A |
| Ref : Phytomedicine , 23 :979 , 2016 |
| Abstract : Uysal_2016_Phytomedicine_23_979 |
| ESTHER : Uysal_2016_Phytomedicine_23_979 |
| PubMedSearch : Uysal_2016_Phytomedicine_23_979 |
| PubMedID: 27444342 |
| Title : Fast identification of lipase inhibitors in oolong tea by using lipase functionalised Fe3O4 magnetic nanoparticles coupled with UPLC-MS\/MS - Zhu_2015_Food.Chem_173_521 |
| Author(s) : Zhu YT , Ren XY , Yuan L , Liu YM , Liang J , Liao X |
| Ref : Food Chem , 173 :521 , 2015 |
| Abstract : Zhu_2015_Food.Chem_173_521 |
| ESTHER : Zhu_2015_Food.Chem_173_521 |
| PubMedSearch : Zhu_2015_Food.Chem_173_521 |
| PubMedID: 25466054 |
| Title : (-)-Epigallocatechin-3-gallate inhibits pancreatic lipase and reduces body weight gain in high fat-fed obese mice - Grove_2012_Obesity.(Silver.Spring)_20_2311 |
| Author(s) : Grove KA , Sae-tan S , Kennett MJ , Lambert JD |
| Ref : Obesity (Silver Spring) , 20 :2311 , 2012 |
| Abstract : Grove_2012_Obesity.(Silver.Spring)_20_2311 |
| ESTHER : Grove_2012_Obesity.(Silver.Spring)_20_2311 |
| PubMedSearch : Grove_2012_Obesity.(Silver.Spring)_20_2311 |
| PubMedID: 21633405 |
| Gene_locus related to this paper: human-PNLIP |
| Title : Insulin-like effect of (-)epicatechin on erythrocyte membrane acetylcholinesterase activity in type 2 diabetes mellitus - Rizvi_2001_Clin.Exp.Pharmacol.Physiol_28_776 |
| Author(s) : Rizvi SI , Zaid MA |
| Ref : Clinical & Experimental Pharmacology & Physiology , 28 :776 , 2001 |
| Abstract : Rizvi_2001_Clin.Exp.Pharmacol.Physiol_28_776 |
| ESTHER : Rizvi_2001_Clin.Exp.Pharmacol.Physiol_28_776 |
| PubMedSearch : Rizvi_2001_Clin.Exp.Pharmacol.Physiol_28_776 |
| PubMedID: 11553037 |