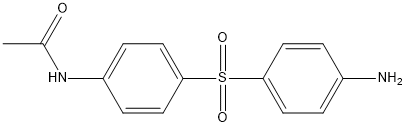
Monoacetyldapsone
General
Type : Acetate, Sulfonyl, Sulfur Compound, Acetanilide
Chemical_Nomenclature : N-[4-(4-aminophenyl)sulfonylphenyl]acetamide
Canonical SMILES : CC(=O)NC1=CC=C(C=C1)S(=O)(=O)C2=CC=C(C=C2)N
InChI : InChI=1S\/C14H14N2O3S\/c1-10(17)16-12-4-8-14(9-5-12)20(18,19)13-6-2-11(15)3-7-13\/h2-9H,15H2,1H3,(H,16,17)
InChIKey : WDOCBIHNYYQINH-UHFFFAOYSA-N
Other name(s) : N-Acetyl Dapsone || N-Acetyldapsone || MADDS
MW : 290.34
Formula : C14H14N2O3S
CAS_number : 565-20-8
PubChem : 11257
UniChem : WDOCBIHNYYQINH-UHFFFAOYSA-N
Target
Structures : No structure
Families : Arylacetamide_deacetylase, Carb_B_Chordata
References (2)
| Title : Comparison of substrate specificity among human arylacetamide deacetylase and carboxylesterases - Fukami_2015_Eur.J.Pharm.Sci_78_47 |
| Author(s) : Fukami T , Kariya M , Kurokawa T , Iida A , Nakajima M |
| Ref : Eur J Pharm Sci , 78 :47 , 2015 |
| Abstract : |
| PubMedSearch : Fukami_2015_Eur.J.Pharm.Sci_78_47 |
| PubMedID: 26164127 |
| Gene_locus related to this paper: human-AADAC , human-CES1 , human-CES2 |
| Title : Arylacetamide deacetylase activity towards monoacetyldapsone. Species comparison, factors that influence activity, and comparison with 2-acetylaminofluorene and p-nitrophenyl acetate hydrolysis - Preuss_1996_Biochem.Pharmacol_51_1661 |
| Author(s) : Preuss CV , Svensson CK |
| Ref : Biochemical Pharmacology , 51 :1661 , 1996 |
| Abstract : |
| PubMedSearch : Preuss_1996_Biochem.Pharmacol_51_1661 |
| PubMedID: 8687481 |