Compound-47
In the screen for inhibitors of ABH2 Compounds 47 and 60 were the most potent ABHD3 inhibitors that remained selective over the other measured
General
Type : Pyridine, Carboxamide
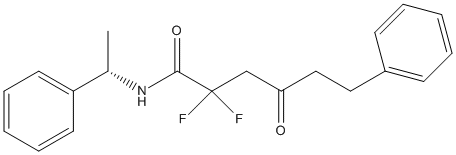
Chemical_Nomenclature : N-(3-Pyridyl)methyl2,2-difluoro-3-oxo-5-phenylvaleramide
Canonical SMILES : O=C(CCc1ccccc1)C(F)(F)C(=O)NCc1cccnc1
InChI : InChI=1S\/C17H16F2N2O2\/c18-17(19,15(22)9-8-13-5-2-1-3-6-13)16(23)21-12-14-7-4-10-20-11-14\/h1-7,10-11H,8-9,12H2,(H,21,23)
InChIKey : BCHBTDIXSOZRCG-UHFFFAOYSA-N
Other name(s) : N-[(pyridin-3-yl)methyl]-2,2-difluoro-3-oxo-5-phenylpentanamide
Target
Families : Compound-47 ligand of proteins in family
abh_upf0017
Protein :
human-ABHD3
References (6)
| Title : alpha\/beta-Hydrolase Domain (ABHD) Inhibitors as New Potential Therapeutic Options against Lipid-Related Diseases - Bononi_2021_J.Med.Chem_64_9759 |
| Author(s) : Bononi G , Tuccinardi T , Rizzolio F , Granchi C |
| Ref : Journal of Medicinal Chemistry , : , 2021 |
| Abstract : |
| PubMedSearch : Bononi_2021_J.Med.Chem_64_9759 |
| PubMedID: 34213320 |
| Gene_locus related to this paper: human-ABHD3 |
| Title : alpha\/beta-Hydrolase Domain (ABHD) Inhibitors as New Potential Therapeutic Options against Lipid-Related Diseases - Bononi_2021_J.Med.Chem_64_9759 |
| Author(s) : Bononi G , Tuccinardi T , Rizzolio F , Granchi C |
| Ref : Journal of Medicinal Chemistry , : , 2021 |
| Abstract : |
| PubMedSearch : Bononi_2021_J.Med.Chem_64_9759 |
| PubMedID: 34213320 |
| Gene_locus related to this paper: human-ABHD3 |
| Title : alpha\/beta-Hydrolase Domain (ABHD) Inhibitors as New Potential Therapeutic Options against Lipid-Related Diseases - Bononi_2021_J.Med.Chem_64_9759 |
| Author(s) : Bononi G , Tuccinardi T , Rizzolio F , Granchi C |
| Ref : Journal of Medicinal Chemistry , : , 2021 |
| Abstract : |
| PubMedSearch : Bononi_2021_J.Med.Chem_64_9759 |
| PubMedID: 34213320 |
| Gene_locus related to this paper: human-ABHD3 |
| Title : ABHD2 Inhibitor Identified by Activity-Based Protein Profiling Reduces Acrosome Reaction - Baggelaar_2019_ACS.Chem.Biol_14_2295 |
| Author(s) : Baggelaar MP , den Dulk H , Florea BI , Fazio D , Perruzza D , Bernabo N , Raspa M , Janssen APA , Scavizzi F , Barboni B , Overkleeft HS , Maccarrone M , van der Stelt M |
| Ref : ACS Chemical Biology , 14 :2295 , 2019 |
| Abstract : |
| PubMedSearch : Baggelaar_2019_ACS.Chem.Biol_14_2295 |
| PubMedID: 31525885 |
| Gene_locus related to this paper: human-ABHD2 , human-ABHD3 |
| Title : ABHD2 Inhibitor Identified by Activity-Based Protein Profiling Reduces Acrosome Reaction - Baggelaar_2019_ACS.Chem.Biol_14_2295 |
| Author(s) : Baggelaar MP , den Dulk H , Florea BI , Fazio D , Perruzza D , Bernabo N , Raspa M , Janssen APA , Scavizzi F , Barboni B , Overkleeft HS , Maccarrone M , van der Stelt M |
| Ref : ACS Chemical Biology , 14 :2295 , 2019 |
| Abstract : |
| PubMedSearch : Baggelaar_2019_ACS.Chem.Biol_14_2295 |
| PubMedID: 31525885 |
| Gene_locus related to this paper: human-ABHD2 , human-ABHD3 |
| Title : ABHD2 Inhibitor Identified by Activity-Based Protein Profiling Reduces Acrosome Reaction - Baggelaar_2019_ACS.Chem.Biol_14_2295 |
| Author(s) : Baggelaar MP , den Dulk H , Florea BI , Fazio D , Perruzza D , Bernabo N , Raspa M , Janssen APA , Scavizzi F , Barboni B , Overkleeft HS , Maccarrone M , van der Stelt M |
| Ref : ACS Chemical Biology , 14 :2295 , 2019 |
| Abstract : |
| PubMedSearch : Baggelaar_2019_ACS.Chem.Biol_14_2295 |
| PubMedID: 31525885 |
| Gene_locus related to this paper: human-ABHD2 , human-ABHD3 |