Gosogliptin
Gosogliptin (SatRx) was developed by Pfizer. It is approved for use in Russia
General
Type : Drug, Piperidine, Pyrimidine, Gliptin
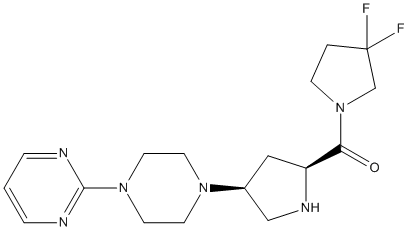
Chemical_Nomenclature : (3,3-difluoropyrrolidin-1-yl)-[(2S,4S)-4-(4-pyrimidin-2-ylpiperazin-1-yl)pyrrolidin-2-yl]methanone
Canonical SMILES : C1CN(CC1(F)F)C(=O)C2CC(CN2)N3CCN(CC3)C4=NC=CC=N4
InChI : InChI=1S\/C17H24F2N6O\/c18-17(19)2-5-25(12-17)15(26)14-10-13(11-22-14)23-6-8-24(9-7-23)16-20-3-1-4-21-16\/h1,3-4,13-14,22H,2,5-12H2\/t13-,14-\/m0\/s1
InChIKey : QWEWGXUTRTXFRF-KBPBESRZSA-N
Other name(s) : UNII-GI718UO477 || PF-00734200 || PF-734200 || CHEMBL515387
MW : 366.41
Formula : C17H24F2N6O
CAS_number : 869490-23-3
PubChem : 11516136
UniChem : QWEWGXUTRTXFRF-KBPBESRZSA-N
Wikipedia : Gosogliptin
Target
Families : Gosogliptin ligand of proteins in family
DPP4N_Peptidase_S9
Structure :
3F8S
Protein :
human-DPP4
References (6)
| Title : The pharmacokinetics of PF-734200, a DPP-IV inhibitor, in subjects with renal insufficiency - Dai_2011_Br.J.Clin.Pharmacol_72_85 |
| Author(s) : Dai H , Johnson SL , Terra SG , Marbury TC , Smith WB , Alcorn H , Boyd RA , Wang R , Nguyen TT |
| Ref : British Journal of Clinical Pharmacology , 72 :85 , 2011 |
| Abstract : |
| PubMedSearch : Dai_2011_Br.J.Clin.Pharmacol_72_85 |
| PubMedID: 21366665 |
| Title : The pharmacokinetics of PF-734200, a DPP-IV inhibitor, in subjects with renal insufficiency - Dai_2011_Br.J.Clin.Pharmacol_72_85 |
| Author(s) : Dai H , Johnson SL , Terra SG , Marbury TC , Smith WB , Alcorn H , Boyd RA , Wang R , Nguyen TT |
| Ref : British Journal of Clinical Pharmacology , 72 :85 , 2011 |
| Abstract : |
| PubMedSearch : Dai_2011_Br.J.Clin.Pharmacol_72_85 |
| PubMedID: 21366665 |
| Title : The pharmacokinetics of PF-734200, a DPP-IV inhibitor, in subjects with renal insufficiency - Dai_2011_Br.J.Clin.Pharmacol_72_85 |
| Author(s) : Dai H , Johnson SL , Terra SG , Marbury TC , Smith WB , Alcorn H , Boyd RA , Wang R , Nguyen TT |
| Ref : British Journal of Clinical Pharmacology , 72 :85 , 2011 |
| Abstract : |
| PubMedSearch : Dai_2011_Br.J.Clin.Pharmacol_72_85 |
| PubMedID: 21366665 |
| Title : (3,3-Difluoro-pyrrolidin-1-yl)-[(2S,4S)-(4-(4-pyrimidin-2-yl-piperazin-1-yl)-pyrr olidin-2-yl]-methanone: a potent, selective, orally active dipeptidyl peptidase IV inhibitor - Ammirati_2009_Bioorg.Med.Chem.Lett_19_1991 |
| Author(s) : Ammirati MJ , Andrews KM , Boyer DD , Brodeur AM , Danley DE , Doran SD , Hulin B , Liu S , McPherson RK , Orena SJ , Parker JC , Polivkova J , Qiu X , Soglia CB , Treadway JL , VanVolkenburg MA , Wilder DC , Piotrowski DW |
| Ref : Bioorganic & Medicinal Chemistry Lett , 19 :1991 , 2009 |
| Abstract : |
| PubMedSearch : Ammirati_2009_Bioorg.Med.Chem.Lett_19_1991 |
| PubMedID: 19275964 |
| Gene_locus related to this paper: human-DPP4 |
| Title : (3,3-Difluoro-pyrrolidin-1-yl)-[(2S,4S)-(4-(4-pyrimidin-2-yl-piperazin-1-yl)-pyrr olidin-2-yl]-methanone: a potent, selective, orally active dipeptidyl peptidase IV inhibitor - Ammirati_2009_Bioorg.Med.Chem.Lett_19_1991 |
| Author(s) : Ammirati MJ , Andrews KM , Boyer DD , Brodeur AM , Danley DE , Doran SD , Hulin B , Liu S , McPherson RK , Orena SJ , Parker JC , Polivkova J , Qiu X , Soglia CB , Treadway JL , VanVolkenburg MA , Wilder DC , Piotrowski DW |
| Ref : Bioorganic & Medicinal Chemistry Lett , 19 :1991 , 2009 |
| Abstract : |
| PubMedSearch : Ammirati_2009_Bioorg.Med.Chem.Lett_19_1991 |
| PubMedID: 19275964 |
| Gene_locus related to this paper: human-DPP4 |
| Title : (3,3-Difluoro-pyrrolidin-1-yl)-[(2S,4S)-(4-(4-pyrimidin-2-yl-piperazin-1-yl)-pyrr olidin-2-yl]-methanone: a potent, selective, orally active dipeptidyl peptidase IV inhibitor - Ammirati_2009_Bioorg.Med.Chem.Lett_19_1991 |
| Author(s) : Ammirati MJ , Andrews KM , Boyer DD , Brodeur AM , Danley DE , Doran SD , Hulin B , Liu S , McPherson RK , Orena SJ , Parker JC , Polivkova J , Qiu X , Soglia CB , Treadway JL , VanVolkenburg MA , Wilder DC , Piotrowski DW |
| Ref : Bioorganic & Medicinal Chemistry Lett , 19 :1991 , 2009 |
| Abstract : |
| PubMedSearch : Ammirati_2009_Bioorg.Med.Chem.Lett_19_1991 |
| PubMedID: 19275964 |
| Gene_locus related to this paper: human-DPP4 |