Meropenem
Meropenem is a broad-spectrum carbapenem antibiotic. It is active against Gram-positive and Gram-negative bacteria. Meropenem exerts its action by penetrating bacterial cells readily and interfering with the synthesis of vital cell wall components, which leads to cell death. Resistance ot the antibiotic is mainly due to lactamases not related to alpha/beta hydrolases
General
Type : Antibiotic, Pyrrolidine, Carboxamide, Drug, Sulfur Compound, Azabicyclo
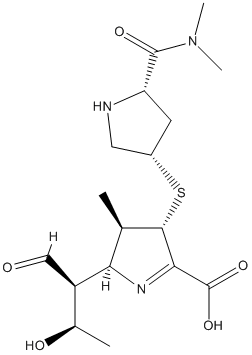
Chemical_Nomenclature : 2S,3R,4S)-4-[(3S,5S)-5-(dimethylcarbamoyl)pyrrolidin-3-yl]sulfanyl-2-[(2S,3R)-3-hydroxy-1-oxobutan-2-yl]-3-methyl-3,4-dihydro-2H-pyrrole-5-carboxylic acid || (4R,5S,6S)-3-[(3S,5S)-5-(dimethylcarbamoyl)pyrrolidin-3-yl]sulfanyl-6-[(1R)-1-hydroxyethyl]-4-methyl-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid
Canonical SMILES : CC1C(C(=NC1C(C=O)C(C)O)C(=O)O)SC2CC(NC2)C(=O)N(C)C || C[C@@H]1[C@@H]2[C@H](C(=O)N2C(=C1S[C@H]3C[C@H](NC3)C(=O)N(C)C)C(=O)O)[C@@H](C)O
InChI : InChI=1S\/C17H27N3O5S\/c1-8-13(11(7-21)9(2)22)19-14(17(24)25)15(8)26-10-5-12(18-6-10)16(23)20(3)4\/h7-13,15,18,22H,5-6H2,1-4H3,(H,24,25)\/t8-,9-,10+,11-,12+,13-,15+\/m1\/s1 || InChI=1S\/C17H25N3O5S\/c1-7-12-11(8(2)21)16(23)20(12)13(17(24)25)14(7)26-9-5-10(18-6-9)15(22)19(3)4\/h7-12,18,21H,5-6H2,1-4H3,(H,24,25)\/t7-,8-,9+,10+,11-,12-\/m1\/s1
InChIKey : UUIYVKJXUXGPKB-VGWSNGFZSA-N || DMJNNHOOLUXYBV-PQTSNVLCSA-N
Other name(s) : (2S,3R,4S)-4-{[(3S,5S)-5-(dimethylcarbamoyl)pyrrolidin-3-yl]sulfanyl}-2-[(2S,3R)-3-hydroxy-1-oxobutan-2-yl]-3-methyl-3,4-dihydro-2H-pyrrole-5-carboxylic acid || Q27459765 || MEPM || (2s,3r,4s)-4-{[(3s,5s)-5-(Dimethylcarbamoyl)pyrrolidin-3-Yl]sulfanyl}-2-[(1s,2r)-1-Formyl-2-Hydroxypropyl]-3-Methyl-3,4-Dihydro-2h-Pyrrole-5-Carboxylic Acid || Merrem || Meropenem anhydrous || Meropenemum || Antibiotic SM 7338 || MERONEM || Merrem I.V. || CHEBI:43968 || SCHEMBL34442 || DB00760
MW : 385.5 || 383.5
Formula : C17H27N3O5S || C17H25N3O5S
CAS_number : 96036-03-2
UniChem : UUIYVKJXUXGPKB-VGWSNGFZSA-N, DMJNNHOOLUXYBV-PQTSNVLCSA-N
Target
Families : Meropenem ligand of proteins in family
ACPH_Peptidase_S9
Protein :
pig-acph
aerpe-APE1547
References (9)
| Title : Ligand binding Pro-miscuity of acylpeptide hydrolase, structural analysis of a detoxifying serine hydrolase - Kiss-Szeman_2025_Protein.Sci_34_e70320 |
| Author(s) : Kiss-Szeman AJ , Takacs L , Jakli I , Banoczi Z , Hosogi N , Traore DAK , Harmat V , Perczel A , Menyhard DK |
| Ref : Protein Science , 34 :e70320 , 2025 |
| Abstract : |
| PubMedSearch : Kiss-Szeman_2025_Protein.Sci_34_e70320 |
| PubMedID: 41074793 |
| Title : Ligand binding Pro-miscuity of acylpeptide hydrolase, structural analysis of a detoxifying serine hydrolase - Kiss-Szeman_2025_Protein.Sci_34_e70320 |
| Author(s) : Kiss-Szeman AJ , Takacs L , Jakli I , Banoczi Z , Hosogi N , Traore DAK , Harmat V , Perczel A , Menyhard DK |
| Ref : Protein Science , 34 :e70320 , 2025 |
| Abstract : |
| PubMedSearch : Kiss-Szeman_2025_Protein.Sci_34_e70320 |
| PubMedID: 41074793 |
| Title : Ligand binding Pro-miscuity of acylpeptide hydrolase, structural analysis of a detoxifying serine hydrolase - Kiss-Szeman_2025_Protein.Sci_34_e70320 |
| Author(s) : Kiss-Szeman AJ , Takacs L , Jakli I , Banoczi Z , Hosogi N , Traore DAK , Harmat V , Perczel A , Menyhard DK |
| Ref : Protein Science , 34 :e70320 , 2025 |
| Abstract : |
| PubMedSearch : Kiss-Szeman_2025_Protein.Sci_34_e70320 |
| PubMedID: 41074793 |
| Title : A carbapenem antibiotic inhibiting a mammalian serine protease: structure of the acylaminoacyl peptidase-meropenem complex - Kiss-Szeman_2022_Chem.Sci_13_14264 |
| Author(s) : Kiss-Szeman AJ , Takacs L , Orgovan Z , Straner P , Jakli I , Schlosser G , Masiulis S , Harmat V , Menyhard DK , Perczel A |
| Ref : Chem Sci , 13 :14264 , 2022 |
| Abstract : |
| PubMedSearch : Kiss-Szeman_2022_Chem.Sci_13_14264 |
| PubMedID: 36545146 |
| Gene_locus related to this paper: pig-acph |
| Title : A carbapenem antibiotic inhibiting a mammalian serine protease: structure of the acylaminoacyl peptidase-meropenem complex - Kiss-Szeman_2022_Chem.Sci_13_14264 |
| Author(s) : Kiss-Szeman AJ , Takacs L , Orgovan Z , Straner P , Jakli I , Schlosser G , Masiulis S , Harmat V , Menyhard DK , Perczel A |
| Ref : Chem Sci , 13 :14264 , 2022 |
| Abstract : |
| PubMedSearch : Kiss-Szeman_2022_Chem.Sci_13_14264 |
| PubMedID: 36545146 |
| Gene_locus related to this paper: pig-acph |
| Title : A carbapenem antibiotic inhibiting a mammalian serine protease: structure of the acylaminoacyl peptidase-meropenem complex - Kiss-Szeman_2022_Chem.Sci_13_14264 |
| Author(s) : Kiss-Szeman AJ , Takacs L , Orgovan Z , Straner P , Jakli I , Schlosser G , Masiulis S , Harmat V , Menyhard DK , Perczel A |
| Ref : Chem Sci , 13 :14264 , 2022 |
| Abstract : |
| PubMedSearch : Kiss-Szeman_2022_Chem.Sci_13_14264 |
| PubMedID: 36545146 |
| Gene_locus related to this paper: pig-acph |
| Title : Characterization of inhibitory effect of carbapenem antibiotics on the deconjugation of valproic acid glucuronide - Masuo_2010_Drug.Metab.Dispos_38_1828 |
| Author(s) : Masuo Y , Ito K , Yamamoto T , Hisaka A , Honma M , Suzuki H |
| Ref : Drug Metabolism & Disposition: The Biological Fate of Chemicals , 38 :1828 , 2010 |
| Abstract : |
| PubMedSearch : Masuo_2010_Drug.Metab.Dispos_38_1828 |
| PubMedID: 20581094 |
| Title : Characterization of inhibitory effect of carbapenem antibiotics on the deconjugation of valproic acid glucuronide - Masuo_2010_Drug.Metab.Dispos_38_1828 |
| Author(s) : Masuo Y , Ito K , Yamamoto T , Hisaka A , Honma M , Suzuki H |
| Ref : Drug Metabolism & Disposition: The Biological Fate of Chemicals , 38 :1828 , 2010 |
| Abstract : |
| PubMedSearch : Masuo_2010_Drug.Metab.Dispos_38_1828 |
| PubMedID: 20581094 |
| Title : Characterization of inhibitory effect of carbapenem antibiotics on the deconjugation of valproic acid glucuronide - Masuo_2010_Drug.Metab.Dispos_38_1828 |
| Author(s) : Masuo Y , Ito K , Yamamoto T , Hisaka A , Honma M , Suzuki H |
| Ref : Drug Metabolism & Disposition: The Biological Fate of Chemicals , 38 :1828 , 2010 |
| Abstract : |
| PubMedSearch : Masuo_2010_Drug.Metab.Dispos_38_1828 |
| PubMedID: 20581094 |