P11149
P11149 competitive, BBB-penetarated weakly, orally active and selective, rapidly hydrolyzed in vivo to yield the potent AChE inhibitor, 6-DMG exhibits an IC50 of 1.3 microM for rat BChE/AChE
General
Type : Adamantyl, Derivative of Galanthamine, Alkaloid, Benzazepin, Azepane, Natural_modified, Organochlorine
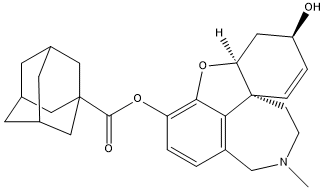
Chemical_Nomenclature : [(1S,12S,14R)-14-hydroxy-4-methyl-11-oxa-4-azatetracyclo[8.6.1.01,12.06,17]heptadeca-6(17),7,9,15-tetraen-9-yl] adamantane-1-carboxylate\;hydrochloride
Canonical SMILES : CN1CCC23C=CC(CC2OC4=C(C=CC(=C34)C1)OC(=O)C56CC7CC(C5)CC(C7)C6)O.Cl
InChI : InChI=1S\/C27H33NO4.ClH\/c1-28-7-6-27-5-4-20(29)11-22(27)32-24-21(3-2-19(15-28)23(24)27)31-25(30)26-12-16-8-17(13-26)10-18(9-16)14-26\;\/h2-5,16-18,20,22,29H,6-15H2,1H3\;1H\/t16?,17?,18?,20-,22-,26?,27-\;\/m0.\/s1
InChIKey : NXIVVAIJLAXRRL-COWQPDHPSA-N
Other name(s) : AKOS040756171 || MS-28734 || HY-105327 || 6-O-demethyl-6-O[(adamantan-1-yl)-carbonyl]galanthamine hydrochloride
Target
Families : P11149 ligand of proteins in family
BCHE
ACHE
Protein :
human-BCHE
human-ACHE
References (3)
| Title : Pharmacological evaluation of novel Alzheimer's disease therapeutics: acetylcholinesterase inhibitors related to galanthamine - Bores_1996_J.Pharmacol.Exp.Ther_277_728 |
| Author(s) : Bores GM , Huger FP , Petko W , Mutlib AE , Camacho F , Rush DK , Selk DE , Wolf V , Kosley RW, Jr. , Davis L , Vargas HM |
| Ref : Journal of Pharmacology & Experimental Therapeutics , 277 :728 , 1996 |
| Abstract : |
| PubMedSearch : Bores_1996_J.Pharmacol.Exp.Ther_277_728 |
| PubMedID: 8627552 |
| Title : Pharmacological evaluation of novel Alzheimer's disease therapeutics: acetylcholinesterase inhibitors related to galanthamine - Bores_1996_J.Pharmacol.Exp.Ther_277_728 |
| Author(s) : Bores GM , Huger FP , Petko W , Mutlib AE , Camacho F , Rush DK , Selk DE , Wolf V , Kosley RW, Jr. , Davis L , Vargas HM |
| Ref : Journal of Pharmacology & Experimental Therapeutics , 277 :728 , 1996 |
| Abstract : |
| PubMedSearch : Bores_1996_J.Pharmacol.Exp.Ther_277_728 |
| PubMedID: 8627552 |
| Title : Pharmacological evaluation of novel Alzheimer's disease therapeutics: acetylcholinesterase inhibitors related to galanthamine - Bores_1996_J.Pharmacol.Exp.Ther_277_728 |
| Author(s) : Bores GM , Huger FP , Petko W , Mutlib AE , Camacho F , Rush DK , Selk DE , Wolf V , Kosley RW, Jr. , Davis L , Vargas HM |
| Ref : Journal of Pharmacology & Experimental Therapeutics , 277 :728 , 1996 |
| Abstract : |
| PubMedSearch : Bores_1996_J.Pharmacol.Exp.Ther_277_728 |
| PubMedID: 8627552 |