XP7-7L4U
General
Type : Piperidine, Pyrrolidine
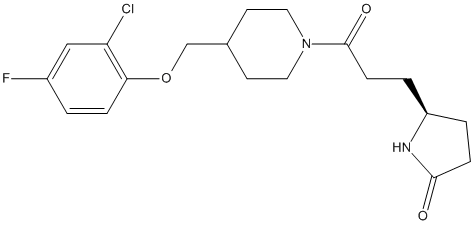
Chemical_Nomenclature : (5S)-5-[3-[4-[(2-chloro-4-fluorophenoxy)methyl]piperidin-1-yl]-3-oxopropyl]pyrrolidin-2-one
Canonical SMILES : C1=C(C=CC(=C1Cl)OCC2CCN(CC2)C(=O)CCC3NC(CC3)=O)F || C1CC(=O)N[C@@H]1CCC(=O)N2CCC(CC2)COC3=C(C=C(C=C3)F)Cl
InChI : InChI=1S\/C19H24ClFN2O3\/c20-16-11-14(21)1-4-17(16)26-12-13-7-9-23(10-8-13)19(25)6-3-15-2-5-18(24)22-15\/h1,4,11,13,15H,2-3,5-10,12H2,(H,22,24)\/t15-\/m0\/s1
InChIKey : AKWBGFHQJGRBNZ-HNNXBMFYSA-N || AKWBGFHQJGRBNZ-UHFFFAOYSA-N
Other name(s) : XP7 || Compound 1h || CHEMBL4867695 || BDBM50566980
MW : 382.86 || 382.9
Formula : C19H24ClFN2O3
CAS_number :
PubChem : 156026020
UniChem : AKWBGFHQJGRBNZ-HNNXBMFYSA-N, AKWBGFHQJGRBNZ-UHFFFAOYSA-N
Target
Families : XP7-7L4U ligand of proteins in family
Monoglyceridelipase_lysophospholip
Structure :
7L4U
Protein :
human-MGLL
References (3)
| Title : Design and Synthesis of Novel Spiro Derivatives as Potent and Reversible Monoacylglycerol Lipase (MAGL) Inhibitors: Bioisosteric Transformation from 3-Oxo-3,4-dihydro-2H-benzo[b][1,4]oxazin-6-yl Moiety - Ikeda_2021_J.Med.Chem__ |
| Author(s) : Ikeda S , Sugiyama H , Tokuhara H , Murakami M , Nakamura M , Oguro Y , Aida J , Morishita N , Sogabe S , Dougan DR , Gay SC , Qin L , Arimura N , Takahashi Y , Sasaki M , Kamada Y , Aoyama K , Kimoto K , Kamata M |
| Ref : Journal of Medicinal Chemistry , : , 2021 |
| Abstract : |
| PubMedSearch : Ikeda_2021_J.Med.Chem__ |
| PubMedID: 34328319 |
| Gene_locus related to this paper: human-MGLL |
| Title : Design and Synthesis of Novel Spiro Derivatives as Potent and Reversible Monoacylglycerol Lipase (MAGL) Inhibitors: Bioisosteric Transformation from 3-Oxo-3,4-dihydro-2H-benzo[b][1,4]oxazin-6-yl Moiety - Ikeda_2021_J.Med.Chem__ |
| Author(s) : Ikeda S , Sugiyama H , Tokuhara H , Murakami M , Nakamura M , Oguro Y , Aida J , Morishita N , Sogabe S , Dougan DR , Gay SC , Qin L , Arimura N , Takahashi Y , Sasaki M , Kamada Y , Aoyama K , Kimoto K , Kamata M |
| Ref : Journal of Medicinal Chemistry , : , 2021 |
| Abstract : |
| PubMedSearch : Ikeda_2021_J.Med.Chem__ |
| PubMedID: 34328319 |
| Gene_locus related to this paper: human-MGLL |
| Title : Design and Synthesis of Novel Spiro Derivatives as Potent and Reversible Monoacylglycerol Lipase (MAGL) Inhibitors: Bioisosteric Transformation from 3-Oxo-3,4-dihydro-2H-benzo[b][1,4]oxazin-6-yl Moiety - Ikeda_2021_J.Med.Chem__ |
| Author(s) : Ikeda S , Sugiyama H , Tokuhara H , Murakami M , Nakamura M , Oguro Y , Aida J , Morishita N , Sogabe S , Dougan DR , Gay SC , Qin L , Arimura N , Takahashi Y , Sasaki M , Kamada Y , Aoyama K , Kimoto K , Kamata M |
| Ref : Journal of Medicinal Chemistry , : , 2021 |
| Abstract : |
| PubMedSearch : Ikeda_2021_J.Med.Chem__ |
| PubMedID: 34328319 |
| Gene_locus related to this paper: human-MGLL |