Fluoride
The fluoride variant of human butyrylcholinesterase owes its name to the observation that it is resistant to inhibition by 0.050 mM sodium fluoride in the in vitro assay. Individuals who are heterozygous for the fluoride and atypical alleles experience about 30 min of apnea, rather than the usual 3-5 min, after receiving succinyldicholine. These mutants correspond to L330I_human-BCHE, T243M_human-BCHE, G390V_human-BCHE Entry of reference human-BCHE
General
Type : Natural,Inorganic chemicals
Chemical_Nomenclature : F-
Canonical SMILES : [F-]
InChI : InChI=1S\/FH\/h1H\/p-1
InChIKey : KRHYYFGTRYWZRS-UHFFFAOYSA-M
Other name(s) : SodiumFluoride,Sodium fluoride,Fluoride,Perfluoride,Fluoride ion,Fluorine, ion
MW : 18.99 || 41.99
Formula : F- || NaF
CAS_number : 16984-48-8 || 7681-49-4
UniChem : KRHYYFGTRYWZRS-UHFFFAOYSA-M
IUPHAR :
Wikipedia : Fluoride
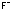
Target
References (13)
| Title : Butyrylcholinesterase genes in individuals with abnormal inhibition numbers and with trace activity: one common mutation and two novel silent genes - Dey_1998_Ann.Clin.Biochem_35_302 |
| Author(s) : Dey DC , Maekawa M , Sudo K , Kanno T |
| Ref : Annals of Clinical Biochemistry , 35 :302 , 1998 |
| Abstract : Dey_1998_Ann.Clin.Biochem_35_302 |
| ESTHER : Dey_1998_Ann.Clin.Biochem_35_302 |
| PubMedSearch : Dey_1998_Ann.Clin.Biochem_35_302 |
| PubMedID: 9547905 |
| Gene_locus related to this paper: human-BCHE |
| Title : Actions of sodium fluoride on acetylcholinesterase activities in rats - Zhao_1998_Biomed.Environ.Sci_11_1 |
| Author(s) : Zhao XL , Wu JH |
| Ref : Biomedical & Environmental Sciences , 11 :1 , 1998 |
| Abstract : Zhao_1998_Biomed.Environ.Sci_11_1 |
| ESTHER : Zhao_1998_Biomed.Environ.Sci_11_1 |
| PubMedSearch : Zhao_1998_Biomed.Environ.Sci_11_1 |
| PubMedID: 9559097 |
| Title : Human butyrylcholinesterase L330I mutation belongs to a fluoride-resistant gene, by expression in human fetal kidney cells - Sudo_1997_Biochem.Biophys.Res.Commun_240_372 |
| Author(s) : Sudo K , Maekawa M , Akizuki S , Magara T , Ogasawara H , Tanaka T |
| Ref : Biochemical & Biophysical Research Communications , 240 :372 , 1997 |
| Abstract : Sudo_1997_Biochem.Biophys.Res.Commun_240_372 |
| ESTHER : Sudo_1997_Biochem.Biophys.Res.Commun_240_372 |
| PubMedSearch : Sudo_1997_Biochem.Biophys.Res.Commun_240_372 |
| PubMedID: 9388484 |
| Gene_locus related to this paper: human-BCHE |
| Title : Identification of human plasma cholinesterase variants in 6,688 individuals using biochemical analysis - Jensen_1995_Acta.Anaesthesiol.Scand_39_157 |
| Author(s) : Jensen FS , Skovgaard LT , Viby-Mogensen J |
| Ref : Acta Anaesthesiologica Scandinavica , 39 :157 , 1995 |
| Abstract : Jensen_1995_Acta.Anaesthesiol.Scand_39_157 |
| ESTHER : Jensen_1995_Acta.Anaesthesiol.Scand_39_157 |
| PubMedSearch : Jensen_1995_Acta.Anaesthesiol.Scand_39_157 |
| PubMedID: 7793180 |
| Title : Recombinant human butyrylcholinesterase G390V, the fluoride-2 variant, expressed in Chinese hamster ovary cells, is a low affinity variant - Masson_1993_J.Biol.Chem_268_14329 |
| Author(s) : Masson P , Adkins S , Gouet P , Lockridge O |
| Ref : Journal of Biological Chemistry , 268 :14329 , 1993 |
| Abstract : Masson_1993_J.Biol.Chem_268_14329 |
| ESTHER : Masson_1993_J.Biol.Chem_268_14329 |
| PubMedSearch : Masson_1993_J.Biol.Chem_268_14329 |
| PubMedID: 8314794 |
| Title : Plasma cholinesterase: gene and variations - Pantuck_1993_Anesth.Analg_77_380 |
| Author(s) : Pantuck EJ |
| Ref : Anesthesia & Analgesia , 77 :380 , 1993 |
| Abstract : Pantuck_1993_Anesth.Analg_77_380 |
| ESTHER : Pantuck_1993_Anesth.Analg_77_380 |
| PubMedSearch : Pantuck_1993_Anesth.Analg_77_380 |
| PubMedID: 8346840 |
| Title : Identification of two different point mutations associated with the fluoride-resistant phenotype for human butyrylcholinesterase - Nogueira_1992_Am.J.Hum.Genet_51_821 |
| Author(s) : Nogueira CP , Bartels CF , McGuire MC , Adkins S , Lubrano T , Rubinstein HM , Lightstone H , van der Spek AF , Lockridge O , La Du BN |
| Ref : American Journal of Human Genetics , 51 :821 , 1992 |
| Abstract : Nogueira_1992_Am.J.Hum.Genet_51_821 |
| ESTHER : Nogueira_1992_Am.J.Hum.Genet_51_821 |
| PubMedSearch : Nogueira_1992_Am.J.Hum.Genet_51_821 |
| PubMedID: 1415224 |
| Title : Malignant hyperthermia and the fluoride-resistant gene - Whittaker_1981_Br.J.Anaesth_53_241 |
| Author(s) : Whittaker M , Britten JJ |
| Ref : British Journal of Anaesthesia , 53 :241 , 1981 |
| Abstract : Whittaker_1981_Br.J.Anaesth_53_241 |
| ESTHER : Whittaker_1981_Br.J.Anaesth_53_241 |
| PubMedSearch : Whittaker_1981_Br.J.Anaesth_53_241 |
| PubMedID: 7470359 |
| Title : Increased sensitivity to succinylcholine in a patient heterozygous for the silent and the fluoride-resistant gene - Viby-Mogensen_1978_Anesth.Analg_57_422 |
| Author(s) : Viby-Mogensen J , Hanel HK |
| Ref : Anesthesia & Analgesia , 57 :422 , 1978 |
| Abstract : Viby-Mogensen_1978_Anesth.Analg_57_422 |
| ESTHER : Viby-Mogensen_1978_Anesth.Analg_57_422 |
| PubMedSearch : Viby-Mogensen_1978_Anesth.Analg_57_422 |
| PubMedID: 568403 |
| Title : The pseudocholinesterase variants. A family segregating for two homozygotes with the fluoride resistant gene - |
| Author(s) : Whittaker M , Berry M |
| Ref : Hum Hered , 22 :243 , 1972 |
| PubMedID: 4562379 |
| Title : The frequency of the fluoride resistant gene in a population of British students - |
| Author(s) : Whittaker M |
| Ref : Acta Genet Stat Med , 18 :563 , 1968 |
| PubMedID: 5756527 |
| Title : Effect of fluoride on the reaction of acetylcholinesterase with dimethylcarbamylcholine - |
| Author(s) : Greenspan CM , Wilson IB |
| Ref : Federation Proceedings , 26 :448 , 1967 |
| PubMedID: |
| Title : Action of fluoride on cholinesterase. I. On the mechanism of inhibition - |
| Author(s) : Heilbronn E |
| Ref : Acta Chemica Scandinavica , 19 :1333 , 1965 |
| PubMedID: 5850143 |