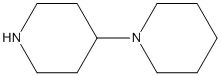
4-piperidino-piperidine
leaving group of hydrolysis of the inhibitor Irinotecan (CPT-11) in mammalian carboxylesterase
General
Type : Leaving group, Derivative of Irinotecan, Piperidine
Chemical_Nomenclature : 1-piperidin-4-ylpiperidine
Canonical SMILES : C1CCN(CC1)C2CCNCC2
InChI : InChI=1S\/C10H20N2\/c1-2-8-12(9-3-1)10-4-6-11-7-5-10\/h10-11H,1-9H2
InChIKey : QDVBKXJMLILLLB-UHFFFAOYSA-N
Other name(s) : 4PN || CHEBI:40117 || CHEMBL174391 || 4-Piperidinopiperidine || 4-Piperidino-piperidine || 1,4'-Bipiperidine || 1,4'-Bipiperidyl
MW : 168.28
Formula : C10H20N2
CAS_number : 4897-50-1
PubChem : 78607
UniChem : QDVBKXJMLILLLB-UHFFFAOYSA-N
Target
Families : 4-piperidino-piperidine ligand of proteins in family
Carb_B_Chordata
Structure :
1K4Y
Protein :
rabit-1cxes
References (1)
| Title : Structural insights into CPT-11 activation by mammalian carboxylesterases - Bencharit_2002_Nat.Struct.Biol_9_337 |
| Author(s) : Bencharit S , Morton CL , Howard-Williams EL , Danks MK , Potter PM , Redinbo MR |
| Ref : Nat Struct Biol , 9 :337 , 2002 |
| Abstract : |
| PubMedSearch : Bencharit_2002_Nat.Struct.Biol_9_337 |
| PubMedID: 11967565 |
| Gene_locus related to this paper: rabit-1cxes |