Jatrorrhizine
very close to Palmitine and Berberine
General
Type : Natural, Alkaloid, Not AlphaBeta Hydrolase target, Isoquinoline, Derivative of berberine
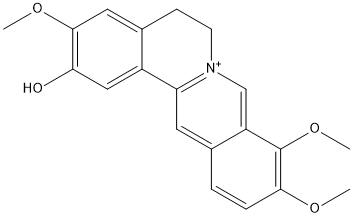
Chemical_Nomenclature : 2,9,10-trimethoxy-5,6-dihydroisoquinolino[2,1-b]isoquinolin-7-ium-3-ol
Canonical SMILES : COC1=C(C2=C[N+]3=C(C=C2C=C1)C4=CC(=C(C=C4CC3)O)OC)OC
InChI : InChI=1S\/C20H19NO4\/c1-23-18-5-4-12-8-16-14-10-19(24-2)17(22)9-13(14)6-7-21(16)11-15(12)20(18)25-3\/h4-5,8-11H,6-7H2,1-3H3\/p+1
InChIKey : MXTLAHSTUOXGQF-UHFFFAOYSA-O
Other name(s) : neprotin || jateorrhizine || Yatrorizine || CHEBI:6087 || CHEMBL251055 || Dehydrocorypalmine || SCHEMBL564128
MW : 338.4
Formula : C20H20NO4+
CAS_number : 3621-38-3
PubChem : 72323
UniChem : MXTLAHSTUOXGQF-UHFFFAOYSA-O
Target
Families : Jatrorrhizine ligand of proteins in family
ACHE
Protein :
human-ACHE
References (13)
| Title : Inhibitory Activity of Quaternary Isoquinoline Alkaloids on Soluble Epoxide Hydrolase - Kim_2022_Curr.Issues.Mol.Biol_44_4282 |
| Author(s) : Kim JH , Cho CW , Hur M , Park WT , Moon YH , Koo SC , Hur YC , Kang JS , Lee IS |
| Ref : Curr Issues Mol Biol , 44 :4282 , 2022 |
| Abstract : |
| PubMedSearch : Kim_2022_Curr.Issues.Mol.Biol_44_4282 |
| PubMedID: 36135206 |
| Title : Simultaneous screening, identification, quantitation, and activity evaluation of six acetylcholinesterase (AChE) inhibitors in Coptidis Rhizoma by online UPLC-DAD coupled with AChE biochemical detection - Tan_2022_J.Pharm.Biomed.Anal_219_114897 |
| Author(s) : Tan JL , Xu YL , Fei YQ , Zheng GH , Ding XP |
| Ref : J Pharm Biomed Anal , 219 :114897 , 2022 |
| Abstract : |
| PubMedSearch : Tan_2022_J.Pharm.Biomed.Anal_219_114897 |
| PubMedID: 35780528 |
| Title : Isolation, derivatization, in-vitro, and in-silico studies of potent butyrylcholinesterase inhibitors from Berberis parkeriana Schneid - Ali_2022_Bioorg.Chem_127_105944 |
| Author(s) : Ali R , Atia Tul W , Wajid S , Khan MA , Yousuf S , Shaikh M , Hassan Laghari G , Rahman AU , Choudhary MI |
| Ref : Bioorg Chem , 127 :105944 , 2022 |
| Abstract : |
| PubMedSearch : Ali_2022_Bioorg.Chem_127_105944 |
| PubMedID: 35905644 |
| Title : A strategy to discover lead chemome from traditional Chinese medicines based on natural chromatogram-effect correlation (NCEC) and natural structure-effect correlation (NSEC): Mahonia bealei and Mahonia fortunei as a case study - Song_2021_J.Chromatogr.B.Analyt.Technol.Biomed.Life.Sci_1181_122922 |
| Author(s) : Song HP , Zhang H , Hu R , Xiao HH , Guo H , Yuan WH , Han XT , Xu XY , Zhang X , Ding ZX , Zhao MY , Kang TG , Sun HY , Chang A , Chen YH , Xie M |
| Ref : Journal of Chromatography B Analyt Technol Biomed Life Sciences , 1181 :122922 , 2021 |
| Abstract : |
| PubMedSearch : Song_2021_J.Chromatogr.B.Analyt.Technol.Biomed.Life.Sci_1181_122922 |
| PubMedID: 34500403 |
| Title : UHPLC With On-Line Coupled Biochemical Detection for High Throughput Screening of Acetylcholinesterase Inhibitors in Coptidis Rhizoma and Cortex Phellodendri - Tan_2021_J.Chromatogr.Sci__ |
| Author(s) : Tan J , Zhang X , Fang J , Shen H , Ding X , Zheng G |
| Ref : Journal of Chromatography Sci , : , 2021 |
| Abstract : |
| PubMedSearch : Tan_2021_J.Chromatogr.Sci__ |
| PubMedID: 34664067 |
| Title : Rhizoma Coptidis for Alzheimer's Disease and Vascular Dementia: A Literature Review - Wang_2020_Curr.Vasc.Pharmacol_18_358 |
| Author(s) : Wang Z , Yang Y , Liu M , Wei Y , Liu J , Pei H , Li H |
| Ref : Curr Vasc Pharmacol , 18 :358 , 2020 |
| Abstract : |
| PubMedSearch : Wang_2020_Curr.Vasc.Pharmacol_18_358 |
| PubMedID: 31291876 |
| Title : Large-scale separation of acetylcholinesterase inhibitors from Zanthoxylum nitidum by pH-zone-refining counter-current chromatography target-guided by ultrafiltration high-performance liquid chromatography with ultraviolet and mass spectrometry screening - Liu_2019_J.Sep.Sci_42_1194 |
| Author(s) : Liu M , Liu Q , Chen M , Huang X , Chen X |
| Ref : J Sep Sci , 42 :1194 , 2019 |
| Abstract : |
| PubMedSearch : Liu_2019_J.Sep.Sci_42_1194 |
| PubMedID: 30638299 |
| Title : Screening of acetylcholinesterase inhibitors and characterizing of phytochemical constituents from Dichocarpum auriculatum (Franch.) W.T. Wang & P. K. Hsiao through UPLC-MS combined with an acetylcholinesterase inhibition assay in vitro - Li_2019_J.Ethnopharmacol_245_112185 |
| Author(s) : Li P , Liu S , Liu Q , Shen J , Yang R , Jiang B , He C , Xiao P |
| Ref : J Ethnopharmacol , 245 :112185 , 2019 |
| Abstract : |
| PubMedSearch : Li_2019_J.Ethnopharmacol_245_112185 |
| PubMedID: 31446073 |
| Title : Online screening of acetylcholinesterase inhibitors in natural products using monolith-based immobilized capillary enzyme reactors combined with liquid chromatography-mass spectrometry - Wang_2018_J.Chromatogr.A_1563_135 |
| Author(s) : Wang L , Zhao Y , Zhang Y , Zhang T , Kool J , Somsen GW , Wang Q , Jiang Z |
| Ref : Journal of Chromatography A , 1563 :135 , 2018 |
| Abstract : |
| PubMedSearch : Wang_2018_J.Chromatogr.A_1563_135 |
| PubMedID: 29866504 |
| Title : An in vitro AChE inhibition assay combined with UF-HPLC-ESI-Q-TOF\/MS approach for screening and characterizing of AChE inhibitors from roots of Coptis chinensis Franch - Zhao_2015_J.Pharm.Biomed.Anal_120_235 |
| Author(s) : Zhao H , Zhou S , Zhang M , Feng J , Wang S , Wang D , Geng Y , Wang X |
| Ref : J Pharm Biomed Anal , 120 :235 , 2015 |
| Abstract : |
| PubMedSearch : Zhao_2015_J.Pharm.Biomed.Anal_120_235 |
| PubMedID: 26760241 |
| Title : [Screening of the active ingredients in natural products by capillary electrophoresis and high performance liquid chromatography-mass spectrometry] - Zhang_2013_Se.Pu_31_640 |
| Author(s) : Zhang Y , Kang J |
| Ref : Se Pu , 31 :640 , 2013 |
| Abstract : |
| PubMedSearch : Zhang_2013_Se.Pu_31_640 |
| PubMedID: 24164032 |
| Title : Acetylcholinesterase inhibitors from Stephania venosa tuber - Ingkaninan_2006_J.Pharm.Pharmacol_58_695 |
| Author(s) : Ingkaninan K , Phengpa P , Yuenyongsawad S , Khorana N |
| Ref : J Pharm Pharmacol , 58 :695 , 2006 |
| Abstract : |
| PubMedSearch : Ingkaninan_2006_J.Pharm.Pharmacol_58_695 |
| PubMedID: 16640839 |