A-230-Nerve-agent
Novichok (newcomer/ newbie/ novice, beginner/) is a series of binary chemical weapons developed by the Soviet Union and Russia between 1971 and 1993. Mirzayanov provided the first description of these agents but are somewhat different from the structures identified later.
General
Type : Organophosphate,Chemical Warfare Agent,Novichok
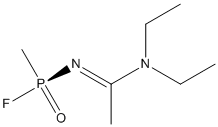
Chemical_Nomenclature : N,N-diethyl-N'-[fluoro(methyl)phosphoryl]ethanimidamide
Canonical SMILES : CCN(CC)C(=NP(=O)(C)F)C
InChI : InChI=1S\/C7H16FN2OP\/c1-5-10(6-2)7(3)9-12(4,8)11\/h5-6H2,1-4H3\/b9-7+\/t12-\/m0\/s1
InChIKey : OUJDIWHRYQBZSR-CRALRDPISA-N
Other name(s) : A-230 (Nerve agent),A230,L2Y
MW : 194.19
Formula : C7H16FN2OP
CAS_number :
PubChem : 146672928
UniChem : OUJDIWHRYQBZSR-CRALRDPISA-N
IUPHAR :
Wikipedia : Novichok_agent
Target
References (10)
| Title : Novichok Nerve Agents as Inhibitors of Acetylcholinesterase-In Silico Study of Their Non-Covalent Binding Affinity - Madaj_2024_Molecules_29_338 |
| Author(s) : Madaj R , Gostynski B , Chworos A , Cypryk M |
| Ref : Molecules , 29 :338 , 2024 |
| Abstract : Madaj_2024_Molecules_29_338 |
| ESTHER : Madaj_2024_Molecules_29_338 |
| PubMedSearch : Madaj_2024_Molecules_29_338 |
| PubMedID: 38257251 |
| Title : Effective Degradation of Novichok Nerve Agents by the Zirconium Metal-Organic Framework MOF-808 - De Koning_2022_ACS.Appl.Mater.Interfaces_14_9222 |
| Author(s) : De Koning MC , Vieira Soares C , van Grol M , Bross RPT , Maurin G |
| Ref : ACS Appl Mater Interfaces , 14 :9222 , 2022 |
| Abstract : De Koning_2022_ACS.Appl.Mater.Interfaces_14_9222 |
| ESTHER : De Koning_2022_ACS.Appl.Mater.Interfaces_14_9222 |
| PubMedSearch : De Koning_2022_ACS.Appl.Mater.Interfaces_14_9222 |
| PubMedID: 35138813 |
| Title : Enzymatic Decontamination of G-Type, V-Type and Novichok Nerve Agents - Jacquet_2021_Int.J.Mol.Sci_22_ |
| Author(s) : Jacquet P , Remy B , Bross RPT , van Grol M , Gaucher F , Chabriere E , De Koning MC , Daude D |
| Ref : Int J Mol Sci , 22 : , 2021 |
| Abstract : Jacquet_2021_Int.J.Mol.Sci_22_ |
| ESTHER : Jacquet_2021_Int.J.Mol.Sci_22_ |
| PubMedSearch : Jacquet_2021_Int.J.Mol.Sci_22_ |
| PubMedID: 34360916 |
| Title : Verification of Exposure to Novichok Nerve Agents Utilizing a Semitargeted Human Butyrylcholinesterase Nonapeptide Assay - Noort_2021_Chem.Res.Toxicol__ |
| Author(s) : Noort D , Fidder A , van der Riet-van Oeveren D , Busker R , van der Schans MJ |
| Ref : Chemical Research in Toxicology , : , 2021 |
| Abstract : Noort_2021_Chem.Res.Toxicol__ |
| ESTHER : Noort_2021_Chem.Res.Toxicol__ |
| PubMedSearch : Noort_2021_Chem.Res.Toxicol__ |
| PubMedID: 34255498 |
| Title : Chemical Properties, Biological Activities and Poisoning Treatment of Novichok: A Review - Rahmania_2021_Pharm.Sci.Res_8_73 |
| Author(s) : Rahmania TA , Bantari Wisynu Kusuma Wardhani BWK , Renesteen E , Harahap Y |
| Ref : Pharmaceutical Sciences and Research (PSR) , 8 :73 , 2021 |
| Abstract : Rahmania_2021_Pharm.Sci.Res_8_73 |
| ESTHER : Rahmania_2021_Pharm.Sci.Res_8_73 |
| PubMedSearch : Rahmania_2021_Pharm.Sci.Res_8_73 |
| PubMedID: |
| Title : Backbone Conformation Shifts in X-ray Structures of Human Acetylcholinesterase upon Covalent Organophosphate Inhibition - Luedtke_2021_Crystals_11_1270 |
| Author(s) : Luedtke S , Bojo C , Li Y , Luna E , Bianca Pomar , Radic Z |
| Ref : Crystals , 11 :1270 , 2021 |
| Abstract : Luedtke_2021_Crystals_11_1270 |
| ESTHER : Luedtke_2021_Crystals_11_1270 |
| PubMedSearch : Luedtke_2021_Crystals_11_1270 |
| PubMedID: |
| Title : Novichoks: The Dangerous Fourth Generation of Chemical Weapons - Franca_2019_Int.J.Mol.Sci_20_ |
| Author(s) : Franca TCC , Kitagawa DAS , Cavalcante SFA , da Silva JAV , Nepovimova E , Kuca K |
| Ref : Int J Mol Sci , 20 : , 2019 |
| Abstract : Franca_2019_Int.J.Mol.Sci_20_ |
| ESTHER : Franca_2019_Int.J.Mol.Sci_20_ |
| PubMedSearch : Franca_2019_Int.J.Mol.Sci_20_ |
| PubMedID: 30862059 |
| Title : Novichok: a murderous nerve agent attack in the UK - |
| Author(s) : Vale JA , Marrs TO , Maynard RC |
| Ref : Clinical Toxicology (Phila) , 56 :1093 , 2018 |
| PubMedID: 29757015 |
| Title : Novichok agents: a historical, current, and toxicological perspective - Chai_2018_Toxicol.Commun_2_45 |
| Author(s) : Chai PR , Hayes BD , Erickson TB , Boyer EW |
| Ref : Toxicol Commun , 2 :45 , 2018 |
| Abstract : Chai_2018_Toxicol.Commun_2_45 |
| ESTHER : Chai_2018_Toxicol.Commun_2_45 |
| PubMedSearch : Chai_2018_Toxicol.Commun_2_45 |
| PubMedID: 30003185 |
| Title : Chemical warfare agent NOVICHOK - mini-review of available data - Nepovimova_2018_Food.Chem.Toxicol_121_343 |
| Author(s) : Nepovimova E , Kuca K |
| Ref : Food & Chemical Toxicology , 121 :343 , 2018 |
| Abstract : Nepovimova_2018_Food.Chem.Toxicol_121_343 |
| ESTHER : Nepovimova_2018_Food.Chem.Toxicol_121_343 |
| PubMedSearch : Nepovimova_2018_Food.Chem.Toxicol_121_343 |
| PubMedID: 30213549 |