MHH
Inhibition by Methyl 4-nitrophenyl hexylphosphonate 6RB0 gives the same adduct MHH (methyl hydrogen (R)-hexylphosphonate) on the active serine as 4-methylumbelliferyl-hexylphosphonate 3ICW
General
Type : Organophosphate,pNP
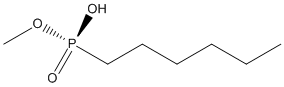
Chemical_Nomenclature : hexyl(methoxy)phosphinic acid
Canonical SMILES : CCCCCCP(=O)(O)OC
InChI : InChI=1S\/C7H17O3P\/c1-3-4-5-6-7-11(8,9)10-2\/h3-7H2,1-2H3,(H,8,9)
InChIKey : FOQQOPHBXMFLMD-UHFFFAOYSA-N
Other name(s) : Methyl Hydrogen (R)-Hexylphosphonate,SCHEMBL14883684,Q27463148
MW : 180.18
Formula : C7H17O3P
CAS_number :
PubChem : 20196937
UniChem : FOQQOPHBXMFLMD-UHFFFAOYSA-N
IUPHAR :
Wikipedia :
Target
Families : MHH ligand of proteins in family: Hormone-sensitive_lipase_like
Stucture : 6RB0 Structure of ester-hydrolase EH1AB1 from the metagenome of lake Arreo complexed with a derivative of methyl 4-nitrophenyl hexylphosphonate
Protein : 9bact-LAE6
References (1)
| Title : Genetically engineered proteins with two active sites for enhanced biocatalysis and synergistic chemo- and biocatalysis - Alonso_2020_Nat.Catal_3_319 |
| Author(s) : Alonso S , Santiago G , Cea-Rama I , Fernandez-Lopez L , Coscolin C , Modregger J , Ressmann A , Martinez-Martinez M , Marrero H , Bargiela R , Pita M , Gonzalez-Alfonso JL , Briand M , Rojo D , Barbas C , Plou FJ , Golyshin PN , Shahgaldian P , Sanz-Aparicio J , Guallar V , Ferrer M |
| Ref : Nature Catalysis , 3 :319 , 2019 |
| Abstract : Alonso_2020_Nat.Catal_3_319 |
| ESTHER : Alonso_2020_Nat.Catal_3_319 |
| PubMedSearch : Alonso_2020_Nat.Catal_3_319 |
| PubMedID: |
| Gene_locus related to this paper: 9bact-LAE6 |