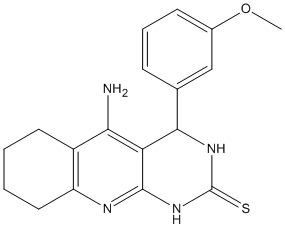
Tacrine-dihydropyrimidine-4e
Juxtaposition of tacrine, as ChE inhibitor (ChEI), and 3,4-dihydropyrimidin-2(1H)-thiones, as efficient calcium channel blockers (CCBs). IC50 3.05 microM and 3.19 microM on hAChE and hBuChE) and moderate CCB (30.4% at 1 micoM) with no significant hepatotoxicity
General
Type : Multitarget,Derivative of Tacrine,Calcium Channel Antagonist,Sulfur Compound,Quinoline,Pyrimidine
Chemical_Nomenclature : 5-amino-4-(3-methoxyphenyl)-3,4,6,7,8,9-hexahydro-1H-pyrimido[4,5-b]quinoline-2-thione
Canonical SMILES : COC1=CC=CC(=C1)C2C3=C(C4=C(CCCC4)N=C3NC(=S)N2)N
InChI : InChI=1S\/C18H20N4OS\/c1-23-11-6-4-5-10(9-11)16-14-15(19)12-7-2-3-8-13(12)20-17(14)22-18(24)21-16\/h4-6,9,16H,2-3,7-8H2,1H3,(H4,19,20,21,22,24)
InChIKey : GLVAOWPNTIXLJA-UHFFFAOYSA-N
Other name(s) : CHEMBL4171520,BDBM50449745
MW : 340.4
Formula : C18H20N4OS
CAS_number :
PubChem : 145950493
UniChem : GLVAOWPNTIXLJA-UHFFFAOYSA-N
IUPHAR :
Wikipedia :
Target
References (1)
| Title : Tacripyrimidines, the first tacrine-dihydropyrimidine hybrids, as multi-target-directed ligands for Alzheimer's disease - Chioua_2018_Eur.J.Med.Chem_155_839 |
| Author(s) : Chioua M , Buzzi E , Moraleda I , Iriepa I , Maj M , Wnorowski A , Giovannini C , Tramarin A , Portali F , Ismaili L , Lopez-Alvarado P , Bolognesi ML , Jozwiak K , Menendez JC , Marco-Contelles J , Bartolini M |
| Ref : Eur Journal of Medicinal Chemistry , 155 :839 , 2018 |
| Abstract : Chioua_2018_Eur.J.Med.Chem_155_839 |
| ESTHER : Chioua_2018_Eur.J.Med.Chem_155_839 |
| PubMedSearch : Chioua_2018_Eur.J.Med.Chem_155_839 |
| PubMedID: 29958119 |