C8-MGDG
General
Type : Acyl-Glycerol || Diacylglycerol || Galactolipid || Glycosydiacylglycerol || Glycoside
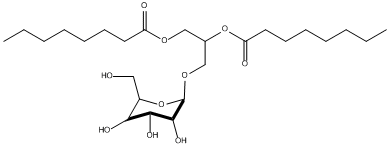
Chemical_Nomenclature : [(2S)-2-octanoyloxy-3-[(2R,3R,4S,5R,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxypropyl] octanoate
Canonical SMILES : CCCCCCCC(=O)OCC(COC1C(C(C(C(O1)CO)O)O)O)OC(=O)CCCCCCC
InChI : InChI=1S\/C25H46O10\/c1-3-5-7-9-11-13-20(27)32-16-18(34-21(28)14-12-10-8-6-4-2)17-33-25-24(31)23(30)22(29)19(15-26)35-25\/h18-19,22-26,29-31H,3-17H2,1-2H3\/t18-,19-,22+,23+,24-,25-\/m1\/s1
InChIKey : UWAHGIGEXYIXRH-IPMCVVNUSA-N
Other name(s) : 1,2-dioctanoyl-3-beta-d-galactosyl-sn-glycerol, SCHEMBL2930610, CHEBI:90453, 1,2-dioctanoyl-3-O-beta-D-galactosyl-sn-glycerol, Q27162558
MW : 506.6
Formula : C25H46O10
CAS_number :
PubChem :
UniChem :
Iuphar :
Target
Families : Pancreatic_lipase, Lipase_3, Cholesterol_esterase, Fungal-Bact_LIP
References (4)
| Title : Enzymatic structural modification of monogalactosyldiacylglycerols for potential modulation of hydrophile-lipophile balance - Roh_2022_Food.Chem_385_132705 |
| Author(s) : Roh S , Lee S , Kim IH , Hee Kim B |
| Ref : Food Chem , 385 :132705 , 2022 |
| Abstract : Roh_2022_Food.Chem_385_132705 |
| ESTHER : Roh_2022_Food.Chem_385_132705 |
| PubMedSearch : Roh_2022_Food.Chem_385_132705 |
| PubMedID: 35306234 |
| Gene_locus related to this paper: rhimi-lipas |
| Title : The galactolipase activity of Fusarium solani (phospho)lipase - Jallouli_2015_Biochim.Biophys.Acta_1851_282 |
| Author(s) : Jallouli R , Othman H , Amara S , Parsiegla G , Carriere F , Srairi-Abid N , Gargouri Y , Bezzine S |
| Ref : Biochimica & Biophysica Acta , 1851 :282 , 2015 |
| Abstract : Jallouli_2015_Biochim.Biophys.Acta_1851_282 |
| ESTHER : Jallouli_2015_Biochim.Biophys.Acta_1851_282 |
| PubMedSearch : Jallouli_2015_Biochim.Biophys.Acta_1851_282 |
| PubMedID: 25529980 |
| Gene_locus related to this paper: nech7-c7yuz1 |
| Title : The galactolipase activity of some microbial lipases and pancreatic enzymes - Amara_2013_Eur.J.Lipid.Sci.Technol_115_442 |
| Author(s) : Amara S , Lafont D , Parsiegla G , Point V , Chabannes A , Rousset A , Carriere F |
| Ref : Eur J Lipid Sci Technol , 115 :442 , 2013 |
| Abstract : Amara_2013_Eur.J.Lipid.Sci.Technol_115_442 |
| ESTHER : Amara_2013_Eur.J.Lipid.Sci.Technol_115_442 |
| PubMedSearch : Amara_2013_Eur.J.Lipid.Sci.Technol_115_442 |
| PubMedID: |
| Gene_locus related to this paper: bovin-balip , human-CEL |
| Title : Inhibition of phospholipase A1, lipase and galactolipase activities of pancreatic lipase-related protein 2 by methyl arachidonyl fluorophosphonate (MAFP) - Amara_2012_Biochim.Biophys.Acta_1821_1379 |
| Author(s) : Amara S , Delorme V , Record M , Carriere F |
| Ref : Biochimica & Biophysica Acta , 1821 :1379 , 2012 |
| Abstract : Amara_2012_Biochim.Biophys.Acta_1821_1379 |
| ESTHER : Amara_2012_Biochim.Biophys.Acta_1821_1379 |
| PubMedSearch : Amara_2012_Biochim.Biophys.Acta_1821_1379 |
| PubMedID: 22835523 |
| Gene_locus related to this paper: human-PNLIPRP2 |