7QB4-Cpd2
(+)-2 MAO-B Ki muM 0.093 +/- 0.015; MAO-B IC50 muM 0.023 +/- 0.003 ; human AChE IC50 muM 1.6 +/- 0.1; mouse AChE IC50 muM 1.5 +/- 0.1 (-)-2 MAO-B Ki muM 0.19 +/- 0.02; MAO-B IC50 muM 0.19 +/- 0.08 ; human AChE IC50 muM 1.3 +/- 0.2; mouse AChE IC50 muM 0.70 +/- 0.05 (+/-)-2 MAO-B Ki muM 0.13 +/- 0.02; MAO-B IC50 muM 0.030 +/- 0.005 ; human AChE IC50 muM 1.4 +/- 0.3
General
Type : Monoamine-oxidase-inhibitor, Multitarget, Coumarin, Chromen, Benzylpiperidine, Derivative of Donepezil
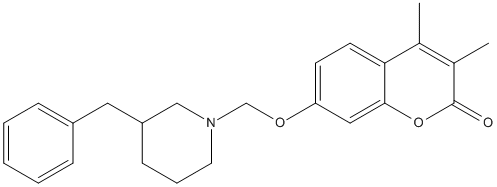
Chemical_Nomenclature : 7-[(1-benzylpiperidin-4-yl)methoxy]-3,4-dimethylchromen-2-one
Canonical SMILES : CC1=C(C(=O)OC2=C1C=CC(=C2)OCC3CCN(CC3)CC4=CC=CC=C4)C
InChI : InChI=1S\/C24H27NO3\/c1-17-18(2)24(26)28-23-14-21(8-9-22(17)23)27-16-20-10-12-25(13-11-20)15-19-6-4-3-5-7-19\/h3-9,14,20H,10-13,15-16H2,1-2H3
InChIKey : MWFBGTXYOJLQIW-UHFFFAOYSA-N
Other name(s) : CHEMBL4598256 || BDBM50585931 || 7-[(1-benzylpiperidin-3-yl)methoxy]-3,4-dimethyl-2H-chromen-2-one
MW : 377.48
Formula : C24H27NO3
CAS_number :
PubChem : 155535905
UniChem : MWFBGTXYOJLQIW-UHFFFAOYSA-N
References (3)
| Title : Dual Reversible Coumarin Inhibitors Mutually Bound to Monoamine Oxidase B and Acetylcholinesterase Crystal Structures - Ekstrom_2022_ACS.Med.Chem.Lett_13_499 |
| Author(s) : Ekstrom F , Gottinger A , Forsgren N , Catto M , Iacovino LG , Pisani P , Binda C |
| Ref : ACS Med Chem Lett , 13 :499 , 2022 |
| Abstract : |
| PubMedSearch : Ekstrom_2022_ACS.Med.Chem.Lett_13_499 |
| PubMedID: 35300078 |
| Gene_locus related to this paper: mouse-ACHE |
| Title : Dual Reversible Coumarin Inhibitors Mutually Bound to Monoamine Oxidase B and Acetylcholinesterase Crystal Structures - Ekstrom_2022_ACS.Med.Chem.Lett_13_499 |
| Author(s) : Ekstrom F , Gottinger A , Forsgren N , Catto M , Iacovino LG , Pisani P , Binda C |
| Ref : ACS Med Chem Lett , 13 :499 , 2022 |
| Abstract : |
| PubMedSearch : Ekstrom_2022_ACS.Med.Chem.Lett_13_499 |
| PubMedID: 35300078 |
| Gene_locus related to this paper: mouse-ACHE |
| Title : Dual Reversible Coumarin Inhibitors Mutually Bound to Monoamine Oxidase B and Acetylcholinesterase Crystal Structures - Ekstrom_2022_ACS.Med.Chem.Lett_13_499 |
| Author(s) : Ekstrom F , Gottinger A , Forsgren N , Catto M , Iacovino LG , Pisani P , Binda C |
| Ref : ACS Med Chem Lett , 13 :499 , 2022 |
| Abstract : |
| PubMedSearch : Ekstrom_2022_ACS.Med.Chem.Lett_13_499 |
| PubMedID: 35300078 |
| Gene_locus related to this paper: mouse-ACHE |