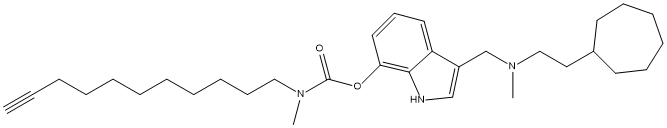
Cpd-17-Cycloheptyl-Indole-undecyl-carbamate
IC50 hBCHE 3.77 nM hACHE 123.2 nM. The alkane moiety terminated by the alkyl is the adduct after inhibition and elimination of the leaving group. It can be used as a bait for click cycloaddition of a probe with an azide-functionalised fluorophore
General
Type : Carbamate, Indole, Cycloheptyl, Derivative of Tryptophan, Alkyne
Chemical_Nomenclature : 3-(((2-Cycloheptylethyl)(methyl)amino)methyl)-1H-indol-7-yl methyl(undec-10-yn-1-yl)carbamate
Canonical SMILES : C12=CC=CC(=C1[N]C=C2CN(CCC3CCCCCC3)C)OC(=O)N(C)CCCCCCCCCC#C
InChI : InChI=1S\/C32H49N3O2\/c1-4-5-6-7-8-9-10-13-16-23-35(3)32(36)37-30-21-17-20-29-28(25-33-31(29)30)26-34(2)24-22-27-18-14-11-12-15-19-27\/h1,17,20-21,25,27,33H,5-16,18-19,22-24,26H2,2-3H3
InChIKey : XVBCFWNSQCHPTI-UHFFFAOYSA-N
Other name(s) :
Target
References (1)
| Title : Pseudo-irreversible butyrylcholinesterase inhibitors: Structure-activity relationships, computational and crystallographic study of the N-dialkyl O-arylcarbamate warhead - Meden_2023_Eur.J.Med.Chem_247_115048 |
| Author(s) : Meden A , Knez D , Brazzolotto X , Modeste F , Perdih A , Pislar A , Zorman M , Zorovic M , Denic M , Pajk S , Zivin M , Nachon F , Gobec S |
| Ref : Eur Journal of Medicinal Chemistry , 247 :115048 , 2023 |
| Abstract : |
| PubMedSearch : Meden_2023_Eur.J.Med.Chem_247_115048 |
| PubMedID: 36586299 |
| Gene_locus related to this paper: human-BCHE |