E0Z
General
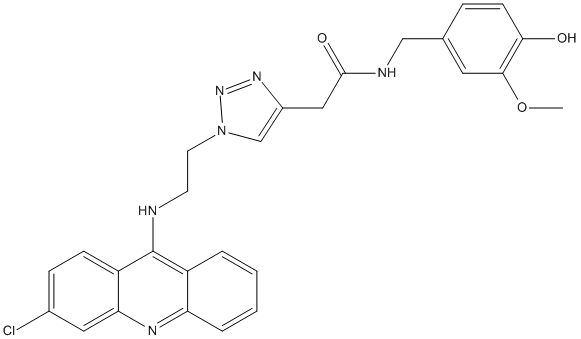
Type : Derivative of Tacrine, Triazol
Chemical_Nomenclature : 2-[1-[2-[(3-chloranylacridin-9-yl)amino]ethyl]-1,2,3-triazol-4-yl]-~{N}-[(3-methoxy-4-oxidanyl-phenyl)methyl]ethanamide\
Canonical SMILES : COc1cc(CNC(=O)Cc2cn(CCNc3c4ccccc4nc4cc(Cl)ccc34)nn2)ccc1O
InChI : InChI=1S\/C27H25ClN6O3\/c1-37-25-12-17(6-9-24(25)35)15-30-26(36)14-19-16-34(33-32-19)11-10-29-27-20-4-2-3-5-22(20)31-23-13-18(28)7-8-21(23)27\/h2-9,12-13,16,35H,10-11,14-15H2,1H3,(H,29,31)(H,30,36)
InChIKey : UQMOCUNKULLJES-UHFFFAOYSA-N
Other name(s) :
MW : 516.98
Formula : C27H25ClN6O3
CAS_number :
PubChem : 132472272
UniChem : UQMOCUNKULLJES-UHFFFAOYSA-N
Target
References (1)
| Title : Increasing Polarity in Tacrine and Huprine Derivatives: Potent Anticholinesterase Agents for the Treatment of Myasthenia Gravis - Galdeano_2018_Molecules_23_ |
| Author(s) : Galdeano C , Coquelle N , Cieslikiewicz-Bouet M , Bartolini M , Perez B , Clos MV , Silman I , Jean L , Colletier JP , Renard PY , Munoz-Torrero D |
| Ref : Molecules , 23 : , 2018 |
| Abstract : |
| PubMedSearch : Galdeano_2018_Molecules_23_ |
| PubMedID: 29534488 |
| Gene_locus related to this paper: torca-ACHE |