40V
merging the scaffold of 8-hydroxyquinoline with that of a known selective butyrylcholinesterase inhibitor. Inhibits with sub-micromolar potency butyrylcholinesterase (IC50=215 nM), and also selectively complexes Cu(2+).
General
Type : Piperidine, Quinoline, Multitarget, Carboxamide, Metal ion chelator, Inden
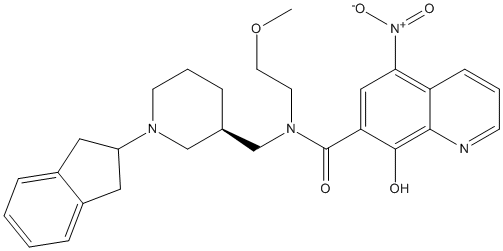
Chemical_Nomenclature : N-[[(3R)-1-(2,3-dihydro-1H-inden-2-yl)piperidin-3-yl]methyl]-8-hydroxy-N-(2-methoxyethyl)-5-nitroquinoline-7-carboxamide
Canonical SMILES : COCCN(CC1CCCN(C1)C2CC3=CC=CC=C3C2)C(=O)C4=CC(=C5C=CC=NC5=C4O)[N+](=O)[O-]
InChI : InChI=1S\/C28H32N4O5\/c1-37-13-12-31(28(34)24-16-25(32(35)36)23-9-4-10-29-26(23)27(24)33)18-19-6-5-11-30(17-19)22-14-20-7-2-3-8-21(20)15-22\/h2-4,7-10,16,19,22,33H,5-6,11-15,17-18H2,1H3\/t19-\/m1\/s1
InChIKey : XTNPQSRETUCLAG-LJQANCHMSA-N
Other name(s) : Compound 8g || N-{[(3r)-1-(2,3-Dihydro-1h-Inden-2-Yl)piperidin-3-Yl]methyl}-8-Hydroxy-N-(2-Methoxyethyl)-5-Nitroquinoline-7-Carboxamide
MW : 504.57
Formula : C28H32N4O5
CAS_number :
PubChem : 91810430
UniChem : XTNPQSRETUCLAG-LJQANCHMSA-N
Target
References (1)
| Title : Structure-based development of nitroxoline derivatives as potential multifunctional anti-Alzheimer agents - Knez_2015_Bioorg.Med.Chem_23_4442 |
| Author(s) : Knez D , Brus B , Coquelle N , Sosic I , Sink R , Brazzolotto X , Mravljak J , Colletier JP , Gobec S |
| Ref : Bioorganic & Medicinal Chemistry , 23 :4442 , 2015 |
| Abstract : |
| PubMedSearch : Knez_2015_Bioorg.Med.Chem_23_4442 |
| PubMedID: 26116179 |
| Gene_locus related to this paper: human-BCHE |