Choline
Product of hydrolysis of acetylcholine by cholinesterases.A basic constituent of lecithin that is found in many plants and animal organs. It is important as a precursor of acetylcholine, as a methyl donor in various metabolic processes, and in lipid metabolism.
General
Type : Natural, Trimethylammonium
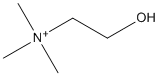
Chemical_Nomenclature : [2-hydroxyethyl]trimethylammonium
Canonical SMILES : C[N+](C)(C)CCO
InChI : InChI=1S\/C5H14NO\/c1-6(2,3)4-5-7\/h7H,4-5H2,1-3H3\/q+1
InChIKey : OEYIOHPDSNJKLS-UHFFFAOYSA-N
Other name(s) : Bilineurine || Hepacholine || Hormocline || Biocolina || Biocoline || Cholinum || Lipotril || Neocolina || Paresan || CHT || CHEBI:15354 || CHEMBL920 || SCHEMBL3142 || ZINC3079337 || DB00122
MW : 104.2
Formula : C5H14NO
CAS_number : 62-49-7
PubChem : 305
UniChem : OEYIOHPDSNJKLS-UHFFFAOYSA-N
Wikipedia : Choline
Target
Families : Choline ligand of proteins in family
ACHE
BCHE
Protein :
mouse-ACHE
human-BCHE
References (6)
| Title : Crystal structure of human butyrylcholinesterase and of its complexes with substrate and products - Nicolet_2003_J.Biol.Chem_278_41141 |
| Author(s) : Nicolet Y , Lockridge O , Masson P , Fontecilla-Camps JC , Nachon F |
| Ref : Journal of Biological Chemistry , 278 :41141 , 2003 |
| Abstract : |
| PubMedSearch : Nicolet_2003_J.Biol.Chem_278_41141 |
| PubMedID: 12869558 |
| Gene_locus related to this paper: human-BCHE |
| Title : Effect of tetramethylammonium, choline and edrophonium on insect acetylcholinesterase: test of a kinetic model - Stojan_1999_Chem.Biol.Interact_119-120_137 |
| Author(s) : Stojan J , Marcel V , Fournier D |
| Ref : Chemico-Biological Interactions , 119-120 :137 , 1999 |
| Abstract : |
| PubMedSearch : Stojan_1999_Chem.Biol.Interact_119-120_137 |
| PubMedID: 10421447 |
| Title : Inhibition of acetylcholinesterase by hemicholiniums, conformationally constrained choline analogues. Evaluation of aryl and alkyl substituents. Comparisons with choline and (3- hydroxyphenyl)trimethylammonium - Lee_1992_Chem.Res.Toxicol_5_411 |
| Author(s) : Lee BH , Stelly TC , Colucci WJ , Garcia JG , Gandour RD , Quinn DM |
| Ref : Chemical Research in Toxicology , 5 :411 , 1992 |
| Abstract : |
| PubMedSearch : Lee_1992_Chem.Res.Toxicol_5_411 |
| PubMedID: 1504265 |
| Title : Genetic variants of human serum cholinesterase influence metabolism of the muscle relaxant succinylcholine. - Lockridge_1990_Pharmacol.Ther_47_35 |
| Author(s) : Lockridge O |
| Ref : Pharmacol Ther , 47 :35 , 1990 |
| Abstract : |
| PubMedSearch : Lockridge_1990_Pharmacol.Ther_47_35 |
| PubMedID: 2195556 |
| Gene_locus related to this paper: human-BCHE |
| Title : Differential inhibition of plasma cholinesterase variants using the dibutyrate analogue of pancuronium bromide - Whittaker_1981_Hum.Hered_31_242 |
| Author(s) : Whittaker M , Britten JJ |
| Ref : Hum Hered , 31 :242 , 1981 |
| Abstract : |
| PubMedSearch : Whittaker_1981_Hum.Hered_31_242 |
| PubMedID: 7287015 |