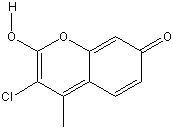
3-chloro-7-hydroxy-4-methylcoumarin
Coumaphos metabolite. Umbelliferone 7-hydroxycoumarins || CHMC is the leaving group of the inhibition of cholinesterases by coumaphos or Haloxon
General
Type : Coumarin
Chemical_Nomenclature : 3-chloro-7-Hydroxy-4-methylcoumarin
Canonical SMILES : CC1=C(C(=O)OC2=C1C=CC(=C2)O)Cl
InChI : InChI=1S\/C10H7ClO3\/c1-5-7-3-2-6(12)4-8(7)14-10(13)9(5)11\/h2-4,12H,1H3
InChIKey : ODZHLDRQCZXQFQ-UHFFFAOYSA-N
Other name(s) : Chlorferone || Chlorferron || 3-Chloro-4-methyl-7-hydroxycoumarin || 7-Hydroxy-4-methyl-3-chlorocoumarin || Coumarin, 3-chloro-7-hydroxy-4-methyl || CHMC || CHEMBL589883 || CHEBI:38620 || SCHEMBL1024770
MW : 210.6
Formula : C10H7ClO3
CAS_number : 6174-86-3
PubChem : 5355079
UniChem : ODZHLDRQCZXQFQ-UHFFFAOYSA-N
References (9)
| Title : Quantification of 3-chloro-7-hydroxy-4-methylcoumarin (CHMC) in urine as a biomarker of coumaphos exposure by high-performance liquid chromatography-fluorescence detection (HPLC-FLD) - Robbins_2025_MethodsX_14_103171 |
| Author(s) : Robbins ZG , Striley CA , Wugofski L |
| Ref : MethodsX , 14 :103171 , 2025 |
| Abstract : |
| PubMedSearch : Robbins_2025_MethodsX_14_103171 |
| PubMedID: 39906117 |
| Title : Computational Design of a Molecularly Imprinted Polymer for the Biomonitoring of the Organophosphorous Metabolite Chlorferron - Qader_2021_Biosensors.(Basel)_11_192 |
| Author(s) : Qader B , Hussain I , Baron M , Jimenez-Perez R , Gil-Ramirez G , Gonzalez-Rodriguez J |
| Ref : Biosensors (Basel) , 11 : , 2021 |
| Abstract : |
| PubMedSearch : Qader_2021_Biosensors.(Basel)_11_192 |
| PubMedID: 34200646 |
| Title : Evaluation of coumaphos exposure among tick eradication workers - Thomas_2010_J.Occup.Environ.Med_52_131 |
| Author(s) : Thomas GA , Delaney LJ , Mueller C , Page E |
| Ref : J Occup Environ Med , 52 :131 , 2010 |
| Abstract : |
| PubMedSearch : Thomas_2010_J.Occup.Environ.Med_52_131 |
| PubMedID: 20134347 |
| Title : Inhibitory potency against human acetylcholinesterase and enzymatic hydrolysis of fluorogenic nerve agent mimics by human paraoxonase 1 and squid diisopropyl fluorophosphatase - Blum_2008_Biochemistry_47_5216 |
| Author(s) : Blum MM , Timperley CM , Williams GR , Thiermann H , Worek F |
| Ref : Biochemistry , 47 :5216 , 2008 |
| Abstract : |
| PubMedSearch : Blum_2008_Biochemistry_47_5216 |
| PubMedID: 18396898 |
| Title : Analogues with fluorescent leaving groups for screening and selection of enzymes that efficiently hydrolyze organophosphorus nerve agents - Briseno-Roa_2006_J.Med.Chem_49_246 |
| Author(s) : Briseno-Roa L , Hill J , Notman S , Sellers D , Smith AP , Timperley CM , Wetherell J , Williams NH , Williams GR , Fersht AR , Griffiths AD |
| Ref : Journal of Medicinal Chemistry , 49 :246 , 2006 |
| Abstract : |
| PubMedSearch : Briseno-Roa_2006_J.Med.Chem_49_246 |
| PubMedID: 16392809 |
| Title : Reversible inhibition of acetylcholinesterase and butyrylcholinesterase by 4,4'-bipyridine and by a coumarin derivative - Simeon-Rudolf_1999_Chem.Biol.Interact_119-120_119 |
| Author(s) : Simeon-Rudolf V , Kovarik Z , Radic Z , Reiner E |
| Ref : Chemico-Biological Interactions , 119-120 :119 , 1999 |
| Abstract : |
| PubMedSearch : Simeon-Rudolf_1999_Chem.Biol.Interact_119-120_119 |
| PubMedID: 10421445 |
| Title : Role of the peripheral anionic site on acetylcholinesterase: inhibition by substrates and coumarin derivatives - Radic_1991_Mol.Pharmacol_39_98 |
| Author(s) : Radic Z , Reiner E , Taylor P |
| Ref : Molecular Pharmacology , 39 :98 , 1991 |
| Abstract : |
| PubMedSearch : Radic_1991_Mol.Pharmacol_39_98 |
| PubMedID: 1987454 |
| Title : Biotransformation and disposition of the coumaphos metabolite 3-chloro-4-methyl-(4-14C)-7-hydroxycoumarin in rats - Malik_1981_Arch.Toxicol_48_51 |
| Author(s) : Malik JK , Lay JP , Klein W , Korte F |
| Ref : Archives of Toxicology , 48 :51 , 1981 |
| Abstract : |
| PubMedSearch : Malik_1981_Arch.Toxicol_48_51 |
| PubMedID: 7283748 |
| Title : Acetylcholinesterase. Two types of inhibition by an organophosphorus compound: one the formation of phosphorylated enzyme and the other analogous to inhibition by substrate - Aldridge_1969_Biochem.J_115_147 |
| Author(s) : Aldridge WN , Reiner E |
| Ref : Biochemical Journal , 115 :147 , 1969 |
| Abstract : |
| PubMedSearch : Aldridge_1969_Biochem.J_115_147 |
| PubMedID: 5378376 |