3F9
nanomolar selective butyrylcholinesterase inhibitor,dissociation constant of 2.7 nM was determined for the most potent stereoisomer (+)-1 structure 4TPK
General
Type : Piperidine, Inden, Carboxamide, Naphthalen
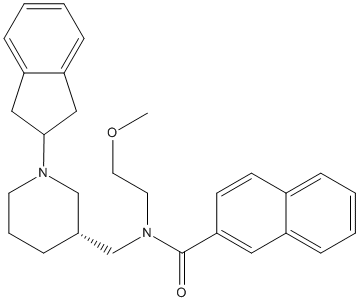
Chemical_Nomenclature : N-[[(3R)-1-(2,3-dihydro-1H-inden-2-yl)piperidin-3-yl]methyl]-N-(2-methoxyethyl)naphthalene-2-carboxamide
Canonical SMILES : COCCN(CC1CCCN(C1)C2CC3=CC=CC=C3C2)C(=O)C4=CC5=CC=CC=C5C=C4
InChI : InChI=1S\/C29H34N2O2\/c1-33-16-15-31(29(32)27-13-12-23-8-2-3-9-24(23)17-27)21-22-7-6-14-30(20-22)28-18-25-10-4-5-11-26(25)19-28\/h2-5,8-13,17,22,28H,6-7,14-16,18-21H2,1H3\/t22-\/m1\/s1
InChIKey : NRBCHFHGWACYAH-JOCHJYFZSA-N
Other name(s) : N-{[(3r)-1-(2,3-Dihydro-1h-Inden-2-Yl)piperidin-3-Yl]methyl}-N-(2-Methoxyethyl)naphthalene-2-Carboxamide || (+)-ZINC-12613047 || ZINC12613047 || CHEMBL3338394
MW : 442.59
Formula : C29H34N2O2
CAS_number :
PubChem : 25396473
UniChem : NRBCHFHGWACYAH-JOCHJYFZSA-N
Target
References (1)
| Title : Discovery, biological evaluation, and crystal structure of a novel nanomolar selective butyrylcholinesterase inhibitor - Brus_2014_J.Med.Chem_57_8167 |
| Author(s) : Brus B , Kosak U , Turk S , Pislar A , Coquelle N , Kos J , Stojan J , Colletier JP , Gobec S |
| Ref : Journal of Medicinal Chemistry , 57 :8167 , 2014 |
| Abstract : |
| PubMedSearch : Brus_2014_J.Med.Chem_57_8167 |
| PubMedID: 25226236 |
| Gene_locus related to this paper: human-BCHE |