IMP-MeCyC
Sarin analogue. Fluorogenic organophosphate diesters that produce fluorescence emission upon hydrolysis. The first ester is ethyl (E), isopropyl (I), cyclohexyl (C) or pinacoyl (P) analogous to the structure of VX, Sarin, Cyclosarin, and Somen respectively. The fluorescent ester is 3-cyano-4-methyl-7-hydroxy coumarin (MeCyC) or 1,3-dichloro-7-hydroxy-9,9-dimethyl-9H-acridin-2-one (DDAO)
General
Type : Organophosphate, Flu-OP, Nerve Agent G-series, Surrogate OP, Derivative of Sarin, Chromen, Coumarin, Cyanide
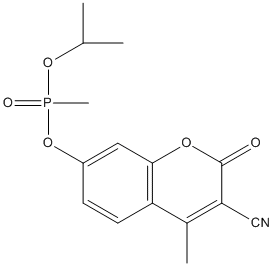
Chemical_Nomenclature : 4-methyl-7-[methyl(propan-2-yloxy)phosphoryl]oxy-2-oxochromene-3-carbonitrile
Canonical SMILES : CC1=C(C(=O)OC2=C1C=CC(=C2)OP(=O)(C)OC(C)C)C#N
InChI : InChI=1S\/C15H16NO5P\/c1-9(2)20-22(4,18)21-11-5-6-12-10(3)13(8-16)15(17)19-14(12)7-11\/h5-7,9H,1-4H3
InChIKey : DQOLUZOTNSTJCY-UHFFFAOYSA-N
Other name(s) : methylphosphonic acid 3-cyano-7-hydroxy-4-mehtyl-2oxo-2H-coumarin-7-yl ester isopropyl ester
MW : 321.27
Formula : C15H16NO5P
CAS_number :
PubChem : 49795036
UniChem : DQOLUZOTNSTJCY-UHFFFAOYSA-N
Target
Families : IMP-MeCyC ligand of proteins in family
ACHE
References (3)
| Title : Resolving pathways of interaction of mipafox and a sarin analog with human acetylcholinesterase by kinetics, mass spectrometry and molecular modeling approaches - Mangas_2016_Arch.Toxicol_90_603 |
| Author(s) : Mangas I , Taylor P , Vilanova E , Estevez J , Franca TCC , Komives E , Radic Z |
| Ref : Archives of Toxicology , 90 :603 , 2016 |
| Abstract : |
| PubMedSearch : Mangas_2016_Arch.Toxicol_90_603 |
| PubMedID: 25743373 |
| Title : Asymmetric fluorogenic organophosphates for the development of active organophosphate hydrolases with reversed stereoselectivity - Amitai_2007_Toxicology_233_187 |
| Author(s) : Amitai G , Adani R , Yacov G , Yishay S , Teitlboim S , Tveria L , Limanovich O , Kushnir M , Meshulam H |
| Ref : Toxicology , 233 :187 , 2007 |
| Abstract : |
| PubMedSearch : Amitai_2007_Toxicology_233_187 |
| PubMedID: 17129656 |
| Title : Enhanced stereoselective hydrolysis of toxic organophosphates by directly evolved variants of mammalian serum paraoxonase - Amitai_2006_FEBS.J_273_1906 |
| Author(s) : Amitai G , Gaidukov L , Adani R , Yishay S , Yacov G , Kushnir M , Teitlboim S , Lindenbaum M , Bel P , Khersonsky O , Tawfik DS , Meshulam H |
| Ref : Febs J , 273 :1906 , 2006 |
| Abstract : |
| PubMedSearch : Amitai_2006_FEBS.J_273_1906 |
| PubMedID: 16640555 |