MGL-Inhibitor-26
Discovery of 1,5-Diphenylpyrazole-3-Carboxamide Derivative MAGL inhibitor (IC50 = 0.51 uM, Ki = 412 nM) with a good selectivity versus fatty acid amide hydrolase (FAAH), alpha/beta-hydrolase domain-containing 6 (ABHD6) and 12 (ABHD12)
General
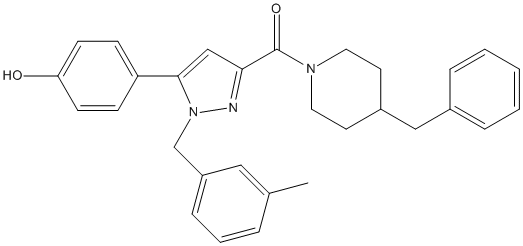
Type : Piperidine, Carboxamide, Pyrazole, Benzylpiperidine, Derivative of Donepezil
Chemical_Nomenclature : (4-Benzylpiperidin-1-yl)(5-(4-hydroxyphenyl)-1-(3-methylbenzyl)-1H-pyrazol-3-yl)methanone
Canonical SMILES : CC1=CC(=CC=C1)CN3C(=C2C=CC(=O)C=C2)C=C(N3)C(=O)N4CCC(CC4)CC5=CC=CC=C5
InChI : InChI=1S\/C30H31N3O2\/c1-22-6-5-9-25(18-22)21-33-29(26-10-12-27(34)13-11-26)20-28(31-33)30(35)32-16-14-24(15-17-32)19-23-7-3-2-4-8-23\/h2-13,18,20,24,31H,14-17,19,21H2,1H3
InChIKey : GZMWVVSFFYCRRT-UHFFFAOYSA-N
Other name(s) : Compound 26 || compound-26
Target
Families : MGL-Inhibitor-26 ligand of proteins in family
Monoglyceridelipase_lysophospholip
Protein :
human-MGLL
References (1)
| Title : Discovery of 1,5-Diphenylpyrazole-3-Carboxamide Derivatives as Potent, Reversible, and Selective Monoacylglycerol Lipase (MAGL) Inhibitors - Aghazadeh Tabrizi_2018_J.Med.Chem_61_1340 |
| Author(s) : Aghazadeh Tabrizi M , Baraldi PG , Baraldi S , Ruggiero E , De Stefano L , Rizzolio F , Di Cesare Mannelli L , Ghelardini C , Chicca A , Lapillo M , Gertsch J , Manera C , Macchia M , Martinelli A , Granchi C , Minutolo F , Tuccinardi T |
| Ref : Journal of Medicinal Chemistry , 61 :1340 , 2018 |
| Abstract : |
| PubMedSearch : Aghazadeh Tabrizi_2018_J.Med.Chem_61_1340 |
| PubMedID: 29309142 |