Phenolic-deriv-Donecopride-20
AChE IC50 6070 +/- 404 nM; 5-HT4R 16% Inhibition at 108 M
General
Type : Carbamate, Multitarget, 5-HT-receptor-ligand, Derivative of Donepezil, Piperidine
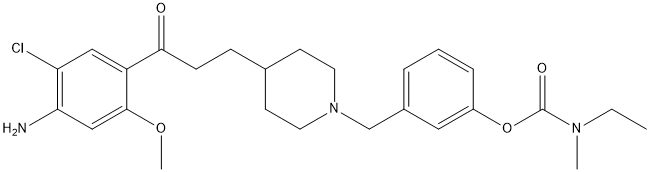
Chemical_Nomenclature : [3-[[4-[3-(4-amino-5-chloro-2-methoxyphenyl)-3-oxopropyl]piperidin-1-yl]methyl]phenyl] N-ethyl-N-methylcarbamate
Canonical SMILES : CCN(C)C(=O)OC1=CC=CC(=C1)CN2CCC(CC2)CCC(=O)C3=CC(=C(C=C3OC)N)Cl
InChI : InChI=1S\/C26H34ClN3O4\/c1-4-29(2)26(32)34-20-7-5-6-19(14-20)17-30-12-10-18(11-13-30)8-9-24(31)21-15-22(27)23(28)16-25(21)33-3\/h5-7,14-16,18H,4,8-13,17,28H2,1-3H3
InChIKey : DLFRLEXQOLTDLP-UHFFFAOYSA-N
Other name(s) : CHEMBL5220228 || BDBM50606812
MW : 488
Formula : C26H34ClN3O4
CAS_number :
PubChem : 168298246
UniChem : DLFRLEXQOLTDLP-UHFFFAOYSA-N
Target
Families : Phenolic-deriv-Donecopride-20 ligand of proteins in family
ACHE
Protein :
human-ACHE
References (1)
| Title : Inhibiting Acetylcholinesterase to Activate Pleiotropic Prodrugs with Therapeutic Interest in Alzheimer's Disease - Toublet_2019_Molecules_24_ |
| Author(s) : Toublet FX , Lecoutey C , Lalut J , Hatat B , Davis A , Since M , Corvaisier S , Freret T , Sopkova de Oliveira Santos J , Claeysen S , Boulouard M , Dallemagne P , Rochais C |
| Ref : Molecules , 24 : , 2019 |
| Abstract : |
| PubMedSearch : Toublet_2019_Molecules_24_ |
| PubMedID: 31370232 |