t-TUCB-PROTAC-P3
General
Type : Urea derivative, Multitarget, FAAH inhibitor, Triazol, Trifluo, Piperidine, Indole, PROTAC
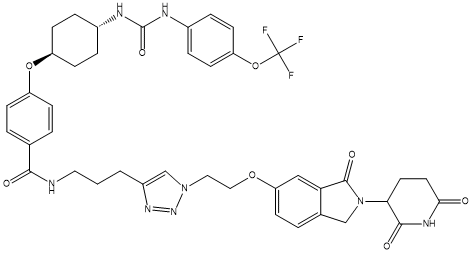
Chemical_Nomenclature : N-(3-(1-(2-((2-(2,6-dioxopiperidin-3-yl)-3-oxoisoindolin-5-yl)oxy)ethyl)-1H-1,2,3-triazol-4-yl)propyl)-4-(((1r,4r)-4-(3-(4-(trifluoromethoxy)phenyl)ureido)cyclohexyl)oxy)benzamide
Canonical SMILES : C1(=CC=C(C=C1)C(=O)NCCCC2=C[N](N=N2)CCOC3=CC=C4C(=C3)C(N(C4)C5C(NC(CC5)=O)=O)=O)O[C@@H]6CC[C@H](CC6)NC(=O)NC7=CC=C(C=C7)OC(F)(F)F
InChI : InChI=1S\/C41H43F3N8O8\/c42-41(43,44)60-32-15-8-28(9-16-32)47-40(57)46-27-6-13-31(14-7-27)59-30-10-3-25(4-11-30)37(54)45-19-1-2-29-24-51(50-49-29)20-21-58-33-12-5-26-23-52(39(56)34(26)22-33)35-17-18-36(53)48-38(35)55\/h3-5,8-12,15-16,22,24,27,31,35H,1-2,6-7,13-14,17-21,23H2,(H,45,54)(H2,46,47,57)(H,48,53,55)\/t27-,31-,35
InChIKey : GPTJTMOOZYWRAR-QYEGHZPBSA-N
Other name(s) : Compound P3
Target
Families : t-TUCB-PROTAC-P3 ligand of proteins in family
Epoxide_hydrolase
Protein :
human-EPHX2
mouse-hyes
References (1)
| Title : Click chemistry enables rapid development of potent sEH PROTACs using a direct-to-biology approach - Schonfeld_2025_Chem.Commun.(Camb)__ |
| Author(s) : Schonfeld J , Liebisch N , Brunst S , Weizel L , Knapp S , Kannt A , Proschak E , Hiesinger K |
| Ref : Chem Commun (Camb) , : , 2025 |
| Abstract : |
| PubMedSearch : Schonfeld_2025_Chem.Commun.(Camb)__ |
| PubMedID: 41120092 |