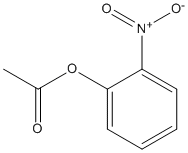
O-Nitrophenylacetate
General
Type : Acetate, Aryl ester, 2-nitrophenyl
Chemical_Nomenclature : (2-nitrophenyl) acetate
Canonical SMILES : CC(=O)OC1=CC=CC=C1[N+](=O)[O-]
InChI : InChI=1S\/C8H7NO4\/c1-6(10)13-8-5-3-2-4-7(8)9(11)12\/h2-5H,1H3
InChIKey : MRCKRGSNLOHYRA-UHFFFAOYSA-N
Other name(s) : ONPA || 2-Nitrophenyl acetate || O-Nitrophenyl acetate || Acetic acid, 2-nitrophenyl ester || 2-Nitrophenol acetate || Acetic acid, o-nitrophenyl ester
MW : 181.1
Formula : C8H7NO4
CAS_number : 610-69-5
PubChem : 11890
UniChem : MRCKRGSNLOHYRA-UHFFFAOYSA-N
Target
Structures : No structure
Families : Carboxylesterase, Cholinesterase, Carb_B_Chordata
References (3)
| Title : Comparison of benzil and trifluoromethyl ketone (TFK)-mediated carboxylesterase inhibition using classical and 3D-quantitative structure-activity relationship analysis - Harada_2009_Bioorg.Med.Chem_17_149 |
| Author(s) : Harada T , Nakagawa Y , Wadkins RM , Potter PM , Wheelock CE |
| Ref : Bioorganic & Medicinal Chemistry , 17 :149 , 2009 |
| Abstract : |
| PubMedSearch : Harada_2009_Bioorg.Med.Chem_17_149 |
| PubMedID: 19062296 |
| Title : Evidence for subdomain flexibility in Drosophila melanogaster acetylcholinesterase - Stojan_2008_Biochemistry_47_5599 |
| Author(s) : Stojan J , Ladurantie C , Siadat OR , Paquereau L , Fournier D |
| Ref : Biochemistry , 47 :5599 , 2008 |
| Abstract : |
| PubMedSearch : Stojan_2008_Biochemistry_47_5599 |
| PubMedID: 18439026 |
| Title : Inhibition of carboxylesterases by benzil (diphenylethane-1,2-dione) and heterocyclic analogues is dependent upon the aromaticity of the ring and the flexibility of the dione moiety - Hyatt_2005_J.Med.Chem_48_5543 |
| Author(s) : Hyatt JL , Stacy V , Wadkins RM , Yoon KJ , Wierdl M , Edwards CC , Zeller M , Hunter AD , Danks MK , Crundwell G , Potter PM |
| Ref : Journal of Medicinal Chemistry , 48 :5543 , 2005 |
| Abstract : |
| PubMedSearch : Hyatt_2005_J.Med.Chem_48_5543 |
| PubMedID: 16107154 |