GT15
preferential AChE/GSK-3beta inhibition (hAChE IC50 = 1.2 +/- 0.1 nM; hGSK-3beta IC50 = 22.2 +/- 1.4 nM).
General
Type : Derivative of Tacrine, Acridine, GSK-3 kinase inhibitor, Sulfur Compound, Multitarget, Carboxamide, Pyridine, Thiazole, Cyclopropyl
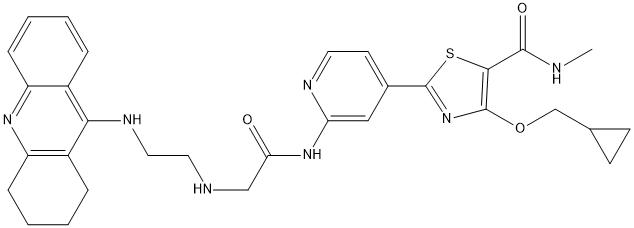
Chemical_Nomenclature : 4-(cyclopropylmethoxy)-N-methyl-2-[2-[[2-[2-(1,2,3,4-tetrahydroacridin-9-ylamino)ethylamino]acetyl]amino]pyridin-4-yl]-1,3-thiazole-5-carboxamide
Canonical SMILES : CNC(=O)C1=C(N=C(S1)C2=CC(=NC=C2)NC(=O)CNCCNC3=C4CCCCC4=NC5=CC=CC=C53)OCC6CC6
InChI : InChI=1S\/C31H35N7O3S\/c1-32-29(40)28-30(41-18-19-10-11-19)38-31(42-28)20-12-13-34-25(16-20)37-26(39)17-33-14-15-35-27-21-6-2-4-8-23(21)36-24-9-5-3-7-22(24)27\/h2,4,6,8,12-13,16,19,33H,3,5,7,9-11,14-15,17-18H2,1H3,(H,32,40)(H,35,36)(H,34,37,39)
InChIKey : MYLYCZDLVLCRLU-UHFFFAOYSA-N
Other name(s) : S0309 || AChE\/GSK-3|A-IN-1 || CHEMBL4789145 || BDBM50556390 || HY-150537 || CS-0534419
MW : 585.7
Formula : C31H35N7O3S
CAS_number :
PubChem : 162669319
UniChem : MYLYCZDLVLCRLU-UHFFFAOYSA-N
Target
Families : GT15 ligand of proteins in family
ACHE
Protein :
human-ACHE
References (3)
| Title : Rational design and biological evaluation of a new class of thiazolopyridyl tetrahydroacridines as cholinesterase and GSK-3 dual inhibitors for Alzheimer's disease - Jiang_2020_Eur.J.Med.Chem_207_112751 |
| Author(s) : Jiang X , Zhou J , Wang Y , Chen L , Duan Y , Huang J , Liu C , Chen Y , Liu W , Sun H , Feng F , Qu W |
| Ref : Eur Journal of Medicinal Chemistry , 207 :112751 , 2020 |
| Abstract : |
| PubMedSearch : Jiang_2020_Eur.J.Med.Chem_207_112751 |
| PubMedID: 32950908 |
| Title : Rational design and biological evaluation of a new class of thiazolopyridyl tetrahydroacridines as cholinesterase and GSK-3 dual inhibitors for Alzheimer's disease - Jiang_2020_Eur.J.Med.Chem_207_112751 |
| Author(s) : Jiang X , Zhou J , Wang Y , Chen L , Duan Y , Huang J , Liu C , Chen Y , Liu W , Sun H , Feng F , Qu W |
| Ref : Eur Journal of Medicinal Chemistry , 207 :112751 , 2020 |
| Abstract : |
| PubMedSearch : Jiang_2020_Eur.J.Med.Chem_207_112751 |
| PubMedID: 32950908 |
| Title : Rational design and biological evaluation of a new class of thiazolopyridyl tetrahydroacridines as cholinesterase and GSK-3 dual inhibitors for Alzheimer's disease - Jiang_2020_Eur.J.Med.Chem_207_112751 |
| Author(s) : Jiang X , Zhou J , Wang Y , Chen L , Duan Y , Huang J , Liu C , Chen Y , Liu W , Sun H , Feng F , Qu W |
| Ref : Eur Journal of Medicinal Chemistry , 207 :112751 , 2020 |
| Abstract : |
| PubMedSearch : Jiang_2020_Eur.J.Med.Chem_207_112751 |
| PubMedID: 32950908 |