JU5
IC50 88nM for human BChE residual activity of 70% mAChE or >74% human AChE. Compound 2C of the paper Pajk et al. derived from inhibitor 5NN0-cpd3 descibed in Kosak et al. 2018
General
Type : Coumarin, Piperidine, Carboxamide, Naphthalen, Sulfur Compound, Thiazole, Chromen
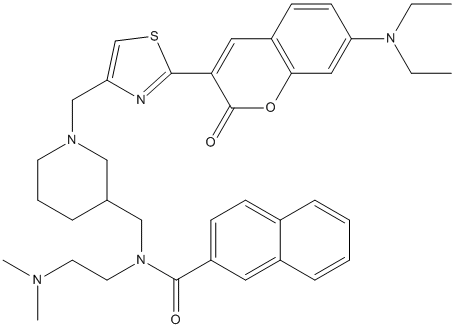
Chemical_Nomenclature : N-((1-((2-(7-(Diethylamino)-2-oxo-2H-chromen-3-yl)thiazol-4-yl)methyl)piperidin-3-yl)methyl)-N-(2-(dimethylamino)ethyl)-2-naphthamide
Canonical SMILES : CCN(CC)C1=CC2=C(C=C1)C=C(C(=O)O2)C3=NC(=CS3)CN4CCCC(C4)CN(CCN(C)C)C(=O)C5=CC6=CC=CC=C6C=C5
InChI : InChI=1S\/C38H45N5O3S\/c1-5-42(6-2)33-16-15-30-21-34(38(45)46-35(30)22-33)36-39-32(26-47-36)25-41-17-9-10-27(23-41)24-43(19-18-40(3)4)37(44)31-14-13-28-11-7-8-12-29(28)20-31\/h7-8,11-16,20-22,26-27H,5-6,9-10,17-19,23-25H2,1-4H3\/t27-\/m0\/s1
InChIKey : GQWCXNBMVVVKQB-MHZLTWQESA-N
Other name(s) : ~{N}-[[(3~{S})-1-[[2-[7-(diethylamino)-2-oxidanylidene-chromen-3-yl]-1,3-thiazol-4-yl]methyl]piperidin-3-yl]methyl]-~{N}-[2-(dimethylamino)ethyl]naphthalene-2-carboxamide || JU5 || 6R6V-JU5
MW : 651.86
Formula : C38H45N5O3S
CAS_number :
PubChem : 145946024
UniChem : GQWCXNBMVVVKQB-MHZLTWQESA-N
Target
References (1)
| Title : Development of potent reversible selective inhibitors of butyrylcholinesterase as fluorescent probes - Pajk_2020_J.Enzyme.Inhib.Med.Chem_35_498 |
| Author(s) : Pajk S , Knez D , Kosak U , Zorovic M , Brazzolotto X , Coquelle N , Nachon F , Colletier JP , Zivin M , Stojan J , Gobec S |
| Ref : J Enzyme Inhib Med Chem , 35 :498 , 2020 |
| Abstract : |
| PubMedSearch : Pajk_2020_J.Enzyme.Inhib.Med.Chem_35_498 |
| PubMedID: 31914836 |
| Gene_locus related to this paper: human-BCHE |