NSD1819
General
Type : Benzodioxo, Piperidine, Triazol, Azetidin
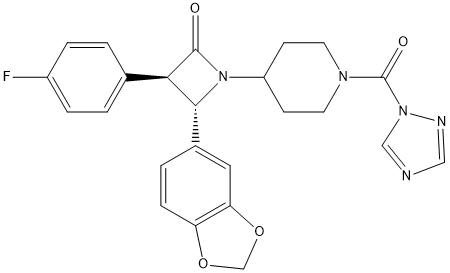
Chemical_Nomenclature : (3R,4S)-4-(1,3-benzodioxol-5-yl)-3-(4-fluorophenyl)-1-[1-(1,2,4-triazole-1-carbonyl)piperidin-4-yl]azetidin-2-one
Canonical SMILES : C1CN(CCC1N2C(C(C2=O)C3=CC=C(C=C3)F)C4=CC5=C(C=C4)OCO5)C(=O)N6C=NC=N6
InChI : InChI=1S\/C24H22FN5O4\/c25-17-4-1-15(2-5-17)21-22(16-3-6-19-20(11-16)34-14-33-19)30(23(21)31)18-7-9-28(10-8-18)24(32)29-13-26-12-27-29\/h1-6,11-13,18,21-22H,7-10,14H2 || InChI=1S\/C24H22FN5O4\/c25-17-4-1-15(2-5-17)21-22(16-3-6-19-20(11-16)34-14-33-19)30(23(21)31)18-7-9-28(10-8-18)24(32)29-13-26-12-27-29\/h1-6,11-13,18,21-22H,7-10,14H2\/t21-,22-\/m1\/s1
InChIKey : XRIROGBLGLPXQI-UHFFFAOYSA-N || XRIROGBLGLPXQI-FGZHOGPDSA-N
Other name(s) : Compound 4a || aka NF1819 || NF 1819 || NF-1819 || 4-(1,3-benzodioxol-5-yl)-3-(4-fluorophenyl)-1-[1-(1,2,4-triazole-1-carbonyl)piperidin-4-yl]azetidin-2-one || MGL-IN-1 || CHEMBL3785379 || (3R,4S)-rel-4-(1,3-Benzodioxol-5-yl)-3-(4-fluorophenyl)-1-[1-(1H)-1,2,4-triazol-1-carbonyl)-4-piperidinyl]-2-azetidinone || CHEMBL3787346
MW : 463.5
Formula : C24H22FN5O4
CAS_number : 1881244-28-5
PubChem : 154731006, 127032456
UniChem : XRIROGBLGLPXQI-UHFFFAOYSA-N, XRIROGBLGLPXQI-FGZHOGPDSA-N
Target
Families : NSD1819 ligand of proteins in family
Monoglyceridelipase_lysophospholip
Protein :
human-MGLL
References (6)
| Title : Inhibition of Monoacylglycerol Lipase by NSD1819 as an Effective Strategy for the Endocannabinoid System Modulation against Neuroinflammation-Related Disorders - Micheli_2022_Int.J.Mol.Sci_23_8428 |
| Author(s) : Micheli L , Maramai S , Toti A , Ferrara V , Ciampi C , Di Cesare Mannelli L , Ghelardini C |
| Ref : Int J Mol Sci , 23 : , 2022 |
| Abstract : |
| PubMedSearch : Micheli_2022_Int.J.Mol.Sci_23_8428 |
| PubMedID: 35955562 |
| Title : Inhibition of Monoacylglycerol Lipase by NSD1819 as an Effective Strategy for the Endocannabinoid System Modulation against Neuroinflammation-Related Disorders - Micheli_2022_Int.J.Mol.Sci_23_8428 |
| Author(s) : Micheli L , Maramai S , Toti A , Ferrara V , Ciampi C , Di Cesare Mannelli L , Ghelardini C |
| Ref : Int J Mol Sci , 23 : , 2022 |
| Abstract : |
| PubMedSearch : Micheli_2022_Int.J.Mol.Sci_23_8428 |
| PubMedID: 35955562 |
| Title : Inhibition of Monoacylglycerol Lipase by NSD1819 as an Effective Strategy for the Endocannabinoid System Modulation against Neuroinflammation-Related Disorders - Micheli_2022_Int.J.Mol.Sci_23_8428 |
| Author(s) : Micheli L , Maramai S , Toti A , Ferrara V , Ciampi C , Di Cesare Mannelli L , Ghelardini C |
| Ref : Int J Mol Sci , 23 : , 2022 |
| Abstract : |
| PubMedSearch : Micheli_2022_Int.J.Mol.Sci_23_8428 |
| PubMedID: 35955562 |
| Title : Development and Pharmacological Characterization of Selective Blockers of 2-Arachidonoyl Glycerol Degradation with Efficacy in Rodent Models of Multiple Sclerosis and Pain - Brindisi_2016_J.Med.Chem_59_2612 |
| Author(s) : Brindisi M , Maramai S , Gemma S , Brogi S , Grillo A , Di Cesare Mannelli L , Gabellieri E , Lamponi S , Saponara S , Gorelli B , Tedesco D , Bonfiglio T , Landry C , Jung KM , Armirotti A , Luongo L , Ligresti A , Piscitelli F , Bertucci C , Dehouck MP , Campiani G , Maione S , Ghelardini C , Pittaluga A , Piomelli D , Di Marzo V , Butini S |
| Ref : Journal of Medicinal Chemistry , 59 :2612 , 2016 |
| Abstract : |
| PubMedSearch : Brindisi_2016_J.Med.Chem_59_2612 |
| PubMedID: 26888301 |
| Title : Development and Pharmacological Characterization of Selective Blockers of 2-Arachidonoyl Glycerol Degradation with Efficacy in Rodent Models of Multiple Sclerosis and Pain - Brindisi_2016_J.Med.Chem_59_2612 |
| Author(s) : Brindisi M , Maramai S , Gemma S , Brogi S , Grillo A , Di Cesare Mannelli L , Gabellieri E , Lamponi S , Saponara S , Gorelli B , Tedesco D , Bonfiglio T , Landry C , Jung KM , Armirotti A , Luongo L , Ligresti A , Piscitelli F , Bertucci C , Dehouck MP , Campiani G , Maione S , Ghelardini C , Pittaluga A , Piomelli D , Di Marzo V , Butini S |
| Ref : Journal of Medicinal Chemistry , 59 :2612 , 2016 |
| Abstract : |
| PubMedSearch : Brindisi_2016_J.Med.Chem_59_2612 |
| PubMedID: 26888301 |
| Title : Development and Pharmacological Characterization of Selective Blockers of 2-Arachidonoyl Glycerol Degradation with Efficacy in Rodent Models of Multiple Sclerosis and Pain - Brindisi_2016_J.Med.Chem_59_2612 |
| Author(s) : Brindisi M , Maramai S , Gemma S , Brogi S , Grillo A , Di Cesare Mannelli L , Gabellieri E , Lamponi S , Saponara S , Gorelli B , Tedesco D , Bonfiglio T , Landry C , Jung KM , Armirotti A , Luongo L , Ligresti A , Piscitelli F , Bertucci C , Dehouck MP , Campiani G , Maione S , Ghelardini C , Pittaluga A , Piomelli D , Di Marzo V , Butini S |
| Ref : Journal of Medicinal Chemistry , 59 :2612 , 2016 |
| Abstract : |
| PubMedSearch : Brindisi_2016_J.Med.Chem_59_2612 |
| PubMedID: 26888301 |