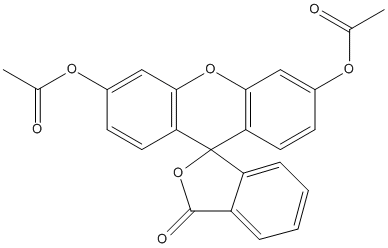
Fluorescein-diacetate
General
Type : Acetate || Fluorescent Probe || Benzofuran || Xanthene
Chemical_Nomenclature : (6'-acetyloxy-3-oxospiro[2-benzofuran-1,9'-xanthene]-3'-yl) acetate
Canonical SMILES : CC(=O)OC1=CC2=C(C=C1)C3(C4=C(O2)C=C(C=C4)OC(=O)C)C5=CC=CC=C5C(=O)O3
InChI : InChI=1S\/C24H16O7\/c1-13(25)28-15-7-9-19-21(11-15)30-22-12-16(29-14(2)26)8-10-20(22)24(19)18-6-4-3-5-17(18)23(27)31-24\/h3-12H,1-2H3
InChIKey : CHADEQDQBURGHL-UHFFFAOYSA-N
Other name(s) : Fluorescein diacetate, 3,6-Diacetoxyfluoran, Fluorescein, diacetate, Di-O-Acetylfluorescein, 3',6'-Diacetylfluorescein
Target
References (14)
| Title : Discovery of pyrazolones as novel carboxylesterase 2 inhibitors that potently inhibit the adipogenesis in cells - Qian_2021_Bioorg.Med.Chem_40_116187 |
| Author(s) : Qian XK , Zhang J , Song PF , Zhao YS , Ma HY , Jin Q , Wang DD , Guan XQ , Li SY , Bao X , Zou LW |
| Ref : Bioorganic & Medicinal Chemistry , 40 :116187 , 2021 |
| Abstract : Qian_2021_Bioorg.Med.Chem_40_116187 |
| ESTHER : Qian_2021_Bioorg.Med.Chem_40_116187 |
| PubMedSearch : Qian_2021_Bioorg.Med.Chem_40_116187 |
| PubMedID: 33965840 |
| Title : Carboxylesterase Activities and Protein Expression in Rabbit and Pig Ocular Tissues - Hammid_2021_Mol.Pharm__ |
| Author(s) : Hammid A , Fallon JK , Lassila T , Salluce G , Smith PC , Tolonen A , Sauer A , Urtti A , Honkakoski P |
| Ref : Mol Pharm , : , 2021 |
| Abstract : Hammid_2021_Mol.Pharm__ |
| ESTHER : Hammid_2021_Mol.Pharm__ |
| PubMedSearch : Hammid_2021_Mol.Pharm__ |
| PubMedID: 33595329 |
| Title : Per- and polyfluoroalkyl substances exert strong inhibition towards human carboxylesterases - Liu_2020_Environ.Pollut_263_114463 |
| Author(s) : Liu YZ , Pan LH , Bai Y , Yang K , Dong PP , Fang ZZ |
| Ref : Environ Pollut , 263 :114463 , 2020 |
| Abstract : Liu_2020_Environ.Pollut_263_114463 |
| ESTHER : Liu_2020_Environ.Pollut_263_114463 |
| PubMedSearch : Liu_2020_Environ.Pollut_263_114463 |
| PubMedID: 32283456 |
| Title : Hydroxylated polychlorinated biphenyls (OH-PCBs) exert strong inhibitory effects towards human carboxylesterases (CESs) - Sun_2020_Sci.Total.Environ_745_141140 |
| Author(s) : Sun HZ , Qin GQ , Wang FG , Bai Y , Zhang Z , Fang ZZ |
| Ref : Sci Total Environ , 745 :141140 , 2020 |
| Abstract : Sun_2020_Sci.Total.Environ_745_141140 |
| ESTHER : Sun_2020_Sci.Total.Environ_745_141140 |
| PubMedSearch : Sun_2020_Sci.Total.Environ_745_141140 |
| PubMedID: 32736114 |
| Title : Presence and inter-individual variability of carboxylesterases (CES1 and CES2) in human lung - Gabriele_2018_Biochem.Pharmacol_150_64 |
| Author(s) : Gabriele M , Puccini P , Lucchi M , Vizziello A , Gervasi PG , Longo V |
| Ref : Biochemical Pharmacology , 150 :64 , 2018 |
| Abstract : Gabriele_2018_Biochem.Pharmacol_150_64 |
| ESTHER : Gabriele_2018_Biochem.Pharmacol_150_64 |
| PubMedSearch : Gabriele_2018_Biochem.Pharmacol_150_64 |
| PubMedID: 29407485 |
| Title : Fluorescein diacetate (FDA) and its analogue as substrates for Pi-class glutathione S-transferase (GSTP1) and their biological application - Fujikawa_2018_Talanta_179_845 |
| Author(s) : Fujikawa Y , Nampo T , Mori M , Kikkawa M , Inoue H |
| Ref : Talanta , 179 :845 , 2018 |
| Abstract : Fujikawa_2018_Talanta_179_845 |
| ESTHER : Fujikawa_2018_Talanta_179_845 |
| PubMedSearch : Fujikawa_2018_Talanta_179_845 |
| PubMedID: 29310316 |
| Title : Heterologous expression, purification and characterization of three novel esterases secreted by the lignocellulolytic fungus Penicillium purpurogenum when grown on sugar beet pulp - Oleas_2017_Carbohydr.Res_443-444_42 |
| Author(s) : Oleas G , Callegari E , Sepulveda R , Eyzaguirre J |
| Ref : Carbohydr Res , 443-444 :42 , 2017 |
| Abstract : Oleas_2017_Carbohydr.Res_443-444_42 |
| ESTHER : Oleas_2017_Carbohydr.Res_443-444_42 |
| PubMedSearch : Oleas_2017_Carbohydr.Res_443-444_42 |
| PubMedID: 28342968 |
| Title : Comparison of substrate specificity among human arylacetamide deacetylase and carboxylesterases - Fukami_2015_Eur.J.Pharm.Sci_78_47 |
| Author(s) : Fukami T , Kariya M , Kurokawa T , Iida A , Nakajima M |
| Ref : Eur J Pharm Sci , 78 :47 , 2015 |
| Abstract : Fukami_2015_Eur.J.Pharm.Sci_78_47 |
| ESTHER : Fukami_2015_Eur.J.Pharm.Sci_78_47 |
| PubMedSearch : Fukami_2015_Eur.J.Pharm.Sci_78_47 |
| PubMedID: 26164127 |
| Gene_locus related to this paper: human-AADAC , human-CES1 , human-CES2 |
| Title : Esterase activity in excised and reconstructed human skin - Biotransformation of prednicarbate and the model dye fluorescein diacetate - Batz_2013_Eur.J.Pharm.Biopharm_84_374 |
| Author(s) : Batz FM , Klipper W , Korting HC , Henkler F , Landsiedel R , Luch A , von Fritschen U , Weindl G , Schafer-Korting M |
| Ref : Eur J Pharm Biopharm , 84 :374 , 2013 |
| Abstract : Batz_2013_Eur.J.Pharm.Biopharm_84_374 |
| ESTHER : Batz_2013_Eur.J.Pharm.Biopharm_84_374 |
| PubMedSearch : Batz_2013_Eur.J.Pharm.Biopharm_84_374 |
| PubMedID: 23201050 |
| Title : Characterization of recombinant human carboxylesterases: fluorescein diacetate as a probe substrate for human carboxylesterase 2 - Wang_2011_Drug.Metab.Dispos_39_1329 |
| Author(s) : Wang J , Williams ET , Bourgea J , Wong YN , Patten CJ |
| Ref : Drug Metabolism & Disposition: The Biological Fate of Chemicals , 39 :1329 , 2011 |
| Abstract : Wang_2011_Drug.Metab.Dispos_39_1329 |
| ESTHER : Wang_2011_Drug.Metab.Dispos_39_1329 |
| PubMedSearch : Wang_2011_Drug.Metab.Dispos_39_1329 |
| PubMedID: 21540359 |
| Gene_locus related to this paper: human-CES2 |
| Title : Application of glutaraldehyde for the staining of esterase-active cells with carboxyfluorescein diacetate - Morono_2004_Biotechnol.Lett_26_379 |
| Author(s) : Morono Y , Takano S , Miyanaga K , Tanji Y , Unno H , Hori K |
| Ref : Biotechnol Lett , 26 :379 , 2004 |
| Abstract : Morono_2004_Biotechnol.Lett_26_379 |
| ESTHER : Morono_2004_Biotechnol.Lett_26_379 |
| PubMedSearch : Morono_2004_Biotechnol.Lett_26_379 |
| PubMedID: 15104134 |
| Title : A three-dimensional flow control concept for single-cell experiments on a microchip. 2. Fluorescein diacetate metabolism and calcium mobilization in a single yeast cell as stimulated by glucose and pH changes - Peng_2004_Anal.Chem_76_5282 |
| Author(s) : Peng XY , Li PC |
| Ref : Analytical Chemistry , 76 :5282 , 2004 |
| Abstract : Peng_2004_Anal.Chem_76_5282 |
| ESTHER : Peng_2004_Anal.Chem_76_5282 |
| PubMedSearch : Peng_2004_Anal.Chem_76_5282 |
| PubMedID: 15362884 |
| Title : Detection of Saccharomyces cerevisiae carboxylesterase activity after native and sodium dodecyl sulfate electrophoresis by using fluorescein diacetate as substrate - Lomolino_2001_Electrophoresis_22_1021 |
| Author(s) : Lomolino G , Lante A , Crapisi A , Spettoli P , Curioni A |
| Ref : Electrophoresis , 22 :1021 , 2001 |
| Abstract : Lomolino_2001_Electrophoresis_22_1021 |
| ESTHER : Lomolino_2001_Electrophoresis_22_1021 |
| PubMedSearch : Lomolino_2001_Electrophoresis_22_1021 |
| PubMedID: 11358123 |
| Title : Membrane properties of living mammalian cells as studied by enzymatic hydrolysis of fluorogenic esters - |
| Author(s) : Rotman B , Papermaster BW |
| Ref : Proc Natl Acad Sci U S A , 55 :134 , 1966 |
| PubMedID: 5220862 |