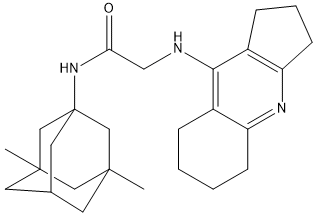
Amiridine-Admantyl-5d
General
Type : Quinoline, Derivative of Tacrine, Derivative of Amiridine, Adamantyl, NMDA-Ligand, Multitarget, Naphthalen
Chemical_Nomenclature : 2-({1H,2H,3H,5H,6H,7H,8H-Cyclopenta[b]naphthalen-4-yl}amino)-N-(3,5-dimethyladamantan-1-yl)acetamide || N-(3,5-dimethyl-1-adamantyl)-2-(2,3,5,6,7,8-hexahydro-1H-cyclopenta[b]quinolin-9-ylamino)acetamide
Canonical SMILES : C1C3(CC2(CC1(CC(C2)C3)NC(CNC4=C6C(=NC5=C4CCC5)CCCC6)=O)C)C || CC12CC3CC(C1)(CC(C3)(C2)NC(=O)CNC4=C5CCCC5=NC6=C4CCCC6)C
InChI : InChI=1S\/C26H37N3O\/c1-24-10-17-11-25(2,14-24)16-26(12-17,15-24)29-22(30)13-27-23-18-6-3-4-8-20(18)28-21-9-5-7-19(21)23\/h17H,3-16H2,1-2H3,(H,27,28)(H,29,30)
InChIKey : DAQUSVFVOAKQLR-UHFFFAOYSA-N
Other name(s) : Compound 5d || BChE-IN-32 || HY-161453 || CS-1049524
MW : 407.59
Formula : C26H37N3O
CAS_number :
PubChem : 171391876
UniChem : DAQUSVFVOAKQLR-UHFFFAOYSA-N
Target
Families : Amiridine-Admantyl-5d ligand of proteins in family
BCHE
Protein :
human-BCHE
References (1)
| Title : Morphing cholinesterase inhibitor amiridine into multipotent drugs for the treatment of Alzheimer's disease - Mezeiova_2024_Biomed.Pharmacother_173_116399 |
| Author(s) : Mezeiova E , Prchal L , Hrabinova M , Muckova L , Pulkrabkova L , Soukup O , Misiachna A , Janousek J , Fibigar J , Kucera T , Horak M , Makhaeva GF , Korabecny J |
| Ref : Biomed Pharmacother , 173 :116399 , 2024 |
| Abstract : |
| PubMedSearch : Mezeiova_2024_Biomed.Pharmacother_173_116399 |
| PubMedID: 38492439 |