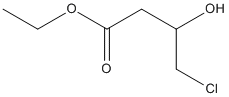
Ethyl-4-chloro-3-hydroxybutanoate
One enzyme convert (S)4-Chloro-3-hydroxybutyrate in the racemate to (S)-3-hydroxy-gamma-butyrolactone through asymmetric dechlorination, hydrolysis, and lactonization. Ethyl (S)-4-chloro-3-hydroxybutyrate is an intermediate for the synthesis of Atorvastatin, a chiral drug used for hypercholesterolemia
General
Type : Alkyl ester, Butyrate
Chemical_Nomenclature : ethyl 4-chloro-3-hydroxybutanoate
Canonical SMILES : CCOC(=O)CC(CCl)O
InChI : InChI=1S\/C6H11ClO3\/c1-2-10-6(9)3-5(8)4-7\/h5,8H,2-4H2,1H3
InChIKey : ZAJNMXDBJKCCAT-UHFFFAOYSA-N
Other name(s) : Ethyl 4-chloro-3-hydroxybutanoate || Ethyl 4-chloro-3-hydroxybutyrate || Butanoic acid, 4-chloro-3-hydroxy-, ethyl ester || Methyl3S-4-chloro-3-hydroxybutanoate || 4-chloro-3-hydroxybutyric acid ethyl ester
MW : 166.60
Formula : C6H11ClO3
CAS_number : 10488-69-4
PubChem : 4377704
UniChem : ZAJNMXDBJKCCAT-UHFFFAOYSA-N
Target
Structures : No structure
Families : Hormone-sensitive_lipase_like
References (2)
| Title : Improvement on production of (R)-4-chloro-3-hydroxybutyrate and (S)-3-hydroxy-gamma-butyrolactone with recombinant Escherichia coli cells - Nakagawa_2006_J.Biosci.Bioeng_101_97 |
| Author(s) : Nakagawa A , Idogaki H , Kato K , Shinmyo A , Suzuki T |
| Ref : J Biosci Bioeng , 101 :97 , 2006 |
| Abstract : |
| PubMedSearch : Nakagawa_2006_J.Biosci.Bioeng_101_97 |
| PubMedID: 16569603 |
| Gene_locus related to this paper: 9entr-q25c43 |