Methyl-salicylate
General
Type : Plant hormone || Natural || Aryl ester || Salicylic || Benzoate
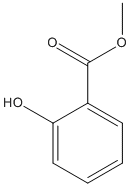
Chemical_Nomenclature : methyl 2-hydroxybenzoate
Canonical SMILES : COC(=O)C1=CC=CC=C1O
InChI : InChI=1S\/C8H8O3\/c1-11-8(10)6-4-2-3-5-7(6)9\/h2-5,9H,1H3
InChIKey : OSWPMRLSEDHDFF-UHFFFAOYSA-N
Other name(s) : MeSA, Methyl salicylate, Methyl 2-hydroxybenzoate, Methyl-2-hydroxybenzoate, Gaultheria oil, Wintergreen oil, Betula oil
Target
Families : Hydroxynitrile_lyase, 6_AlphaBeta_hydrolase
References (5)
| Title : A methyl esterase 1 (PvMES1) promotes the salicylic acid pathway and enhances Fusarium wilt resistance in common beans - Xue_2021_Theor.Appl.Genet__ |
| Author(s) : Xue R , Feng M , Chen J , Ge W , Blair MW |
| Ref : Theor Appl Genet , : , 2021 |
| Abstract : Xue_2021_Theor.Appl.Genet__ |
| ESTHER : Xue_2021_Theor.Appl.Genet__ |
| PubMedSearch : Xue_2021_Theor.Appl.Genet__ |
| PubMedID: 34128089 |
| Gene_locus related to this paper: phavu-v7cf29 |
| Title : An enzyme from Auricularia auricula-judae combining both benzoyl and cinnamoyl esterase activity - Haase-Aschoff_2013_Process.Biochem_48_1872 |
| Author(s) : Haase-Aschoff P , Linke D , Nimtz M , Popper L , Berger RG |
| Ref : Process Biochemistry , 48 :1872 , 2013 |
| Abstract : Haase-Aschoff_2013_Process.Biochem_48_1872 |
| ESTHER : Haase-Aschoff_2013_Process.Biochem_48_1872 |
| PubMedSearch : Haase-Aschoff_2013_Process.Biochem_48_1872 |
| PubMedID: |
| Gene_locus related to this paper: aurst-EJD51015 |
| Title : Inactive methyl indole-3-acetic acid ester can be hydrolyzed and activated by several esterases belonging to the AtMES esterase family of Arabidopsis - Yang_2008_Plant.Physiol_147_1034 |
| Author(s) : Yang Y , Xu R , Ma CJ , Vlot AC , Klessig DF , Pichersky E |
| Ref : Plant Physiol , 147 :1034 , 2008 |
| Abstract : Yang_2008_Plant.Physiol_147_1034 |
| ESTHER : Yang_2008_Plant.Physiol_147_1034 |
| PubMedSearch : Yang_2008_Plant.Physiol_147_1034 |
| PubMedID: 18467465 |
| Gene_locus related to this paper: arath-MES17 , arath-AT4G16690 , arath-AT4G37150 , arath-MES6 , arath-MES7 , arath-MES3 , arath-MES1 , arath-MES18 , arath-MES10 , arath-MES19 |
| Title : Structural and biochemical studies identify tobacco SABP2 as a methyl salicylate esterase and implicate it in plant innate immunity - Forouhar_2005_Proc.Natl.Acad.Sci.U.S.A_102_1773 |
| Author(s) : Forouhar F , Yang Y , Kumar D , Chen Y , Fridman E , Park SW , Chiang Y , Acton TB , Montelione GT , Pichersky E , Klessig DF , Tong L |
| Ref : Proc Natl Acad Sci U S A , 102 :1773 , 2005 |
| Abstract : Forouhar_2005_Proc.Natl.Acad.Sci.U.S.A_102_1773 |
| ESTHER : Forouhar_2005_Proc.Natl.Acad.Sci.U.S.A_102_1773 |
| PubMedSearch : Forouhar_2005_Proc.Natl.Acad.Sci.U.S.A_102_1773 |
| PubMedID: 15668381 |
| Gene_locus related to this paper: nicta-SABP2 |