PGE2-glyceryl-ester
General
Type : Prostaglandin || Acyl-Glycerol || Cannabinoid-Receptor-ligand || Natural
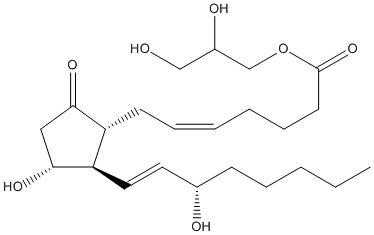
Chemical_Nomenclature : 2,3-dihydroxypropyl (Z)-7-[(1R,2R,3R)-3-hydroxy-2-[(E,3S)-3-hydroxyoct-1-enyl]-5-oxocyclopentyl]hept-5-enoate
Canonical SMILES : CCCCCC(C=CC1C(CC(=O)C1CC=CCCCC(=O)OCC(CO)O)O)O
InChI : InChI=1S\/C23H38O7\/c1-2-3-6-9-17(25)12-13-20-19(21(27)14-22(20)28)10-7-4-5-8-11-23(29)30-16-18(26)15-24\/h4,7,12-13,17-20,22,24-26,28H,2-3,5-6,8-11,14-16H2,1H3\/b7-4-,13-12+\/t17-,18?,19+,20+,22+\/m0\/s1
InChIKey : RJXVYMMSQBYEHN-LVXZDWGESA-N
Other name(s) : PGE2-G, Prostaglandin E2-1-glyceryl ester, Prostaglandin PGE(,2) 1-glycerol, CHEBI:90230, 1(3)-glyceryl-PGE2
Target
References (5)
| Title : Human leukocytes differentially express endocannabinoid-glycerol lipases and hydrolyze 2-arachidonoyl-glycerol and its metabolites from the 15-lipoxygenase and cyclooxygenase pathways - Turcotte_2019_J.Leukoc.Biol_106_1337 |
| Author(s) : Turcotte C , Dumais E , Archambault AS , Martin C , Blanchet MR , Bissonnette E , Boulet LP , Laviolette M , Di Marzo V , Flamand N |
| Ref : J Leukoc Biol , 106 :1337 , 2019 |
| Abstract : Turcotte_2019_J.Leukoc.Biol_106_1337 |
| ESTHER : Turcotte_2019_J.Leukoc.Biol_106_1337 |
| PubMedSearch : Turcotte_2019_J.Leukoc.Biol_106_1337 |
| PubMedID: 31556464 |
| Gene_locus related to this paper: human-ABHD6 , human-ABHD12 , human-ABHD16A , human-CES1 , human-LYPLA2 , human-PPT1 |
| Title : Robust Hydrolysis of Prostaglandin Glycerol Esters by Human Monoacylglycerol Lipase (MAGL) - Savinainen_2014_Mol.Pharmacol_86_522 |
| Author(s) : Savinainen JR , Kansanen E , Pantsar T , Navia-Paldanius D , Parkkari T , Lehtonen M , Laitinen T , Nevalainen T , Poso A , Levonen AL , Laitinen JT |
| Ref : Molecular Pharmacology , 86 :522 , 2014 |
| Abstract : Savinainen_2014_Mol.Pharmacol_86_522 |
| ESTHER : Savinainen_2014_Mol.Pharmacol_86_522 |
| PubMedSearch : Savinainen_2014_Mol.Pharmacol_86_522 |
| PubMedID: 25140003 |
| Gene_locus related to this paper: human-ABHD6 , human-ABHD12 , human-MGLL |
| Title : Possible involvement of brain prostaglandin E2 and prostanoid EP3 receptors in prostaglandin E2 glycerol ester-induced activation of central sympathetic outflow in the rat - Shimizu_2014_Neuropharmacol_82_19 |
| Author(s) : Shimizu T , Tanaka K , Nakamura K , Taniuchi K , Yawata T , Higashi Y , Ueba T , Dimitriadis F , Shimizu S , Yokotani K , Saito M |
| Ref : Neuropharmacology , 82 :19 , 2014 |
| Abstract : Shimizu_2014_Neuropharmacol_82_19 |
| ESTHER : Shimizu_2014_Neuropharmacol_82_19 |
| PubMedSearch : Shimizu_2014_Neuropharmacol_82_19 |
| PubMedID: 24657150 |
| Title : Inactivation of lipid glyceryl ester metabolism in human THP1 monocytes\/macrophages by activated organophosphorus insecticides: role of carboxylesterases 1 and 2 - Xie_2010_Chem.Res.Toxicol_23_1890 |
| Author(s) : Xie S , Borazjani A , Hatfield MJ , Edwards CC , Potter PM , Ross MK |
| Ref : Chemical Research in Toxicology , 23 :1890 , 2010 |
| Abstract : Xie_2010_Chem.Res.Toxicol_23_1890 |
| ESTHER : Xie_2010_Chem.Res.Toxicol_23_1890 |
| PubMedSearch : Xie_2010_Chem.Res.Toxicol_23_1890 |
| PubMedID: 21049984 |
| Title : A physiological role for endocannabinoid-derived products of cyclooxygenase-2-mediated oxidative metabolism - Guindon_2008_Br.J.Pharmacol_153_1341 |
| Author(s) : Guindon J , Hohmann AG |
| Ref : British Journal of Pharmacology , 153 :1341 , 2008 |
| Abstract : Guindon_2008_Br.J.Pharmacol_153_1341 |
| ESTHER : Guindon_2008_Br.J.Pharmacol_153_1341 |
| PubMedSearch : Guindon_2008_Br.J.Pharmacol_153_1341 |
| PubMedID: 18297102 |