Tacrine-Melatonin-Feruloyl-5c
Permeable agent with antioxidant properties,cholinesterase inhibitory activity, less hepatotoxicity than tacrine, and neuroprotective capacity, able to activate the Nrf2 transcriptional pathway. equine BuChE 1.25 and electric eel AChE IC50 3.62 nM
General
Type : Derivative of Tacrine, Derivative of Tryptamine, Indole, Multitarget, Feruloyl, Acrylate, Antioxidant, Hydroxycinnamic-ester, Alkyl linked bis-ligand
Chemical_Nomenclature :
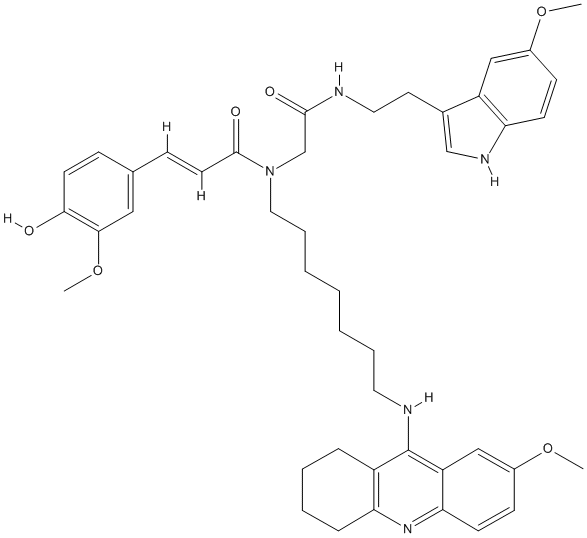
Canonical SMILES : O=C(C(=C(C1=CC(=C(O)C=C1)OC)[H])[H])N(CCCCCCCNC3=C2C=C(OC)C=CC2=NC4=C3CCCC4)CC(NCCC5=C[N]C6=C5C=C(OC)C=C6)=O
InChI : InChI=1S\/C44H53N5O6\/c1-53-32-15-17-37-35(26-32)31(28-47-37)21-23-45-42(51)29-49(43(52)20-14-30-13-19-40(50)41(25-30)55-3)24-10-6-4-5-9-22-46-44-34-11-7-8-12-38(34)48-39-18-16-33(54-2)27-36(39)44\/h13-20,25-28,47,50H,4-12,21-24,29H2,1-3H3,(H,45,51)(H,46,48)\/b20-14+
InChIKey : BCHYGDBEGKZQHD-XSFVSMFZSA-N
Other name(s) :
References (1)
| Title : The Antioxidant Additive Approach for Alzheimer's Disease Therapy: New Ferulic (Lipoic) Acid Plus Melatonin Modified Tacrines as Cholinesterases Inhibitors, Direct Antioxidants, and Nuclear Factor (Erythroid-Derived 2)-Like 2 Activators - Benchekroun_2016_J.Med.Chem_59_9967 |
| Author(s) : Benchekroun M , Romero A , Egea J , Leon R , Michalska P , Buendia I , Jimeno ML , Jun D , Janockova J , Sepsova V , Soukup O , Bautista-Aguilera OM , Refouvelet B , Ouari O , Marco-Contelles J , Ismaili L |
| Ref : Journal of Medicinal Chemistry , 59 :9967 , 2016 |
| Abstract : |
| PubMedSearch : Benchekroun_2016_J.Med.Chem_59_9967 |
| PubMedID: 27736061 |