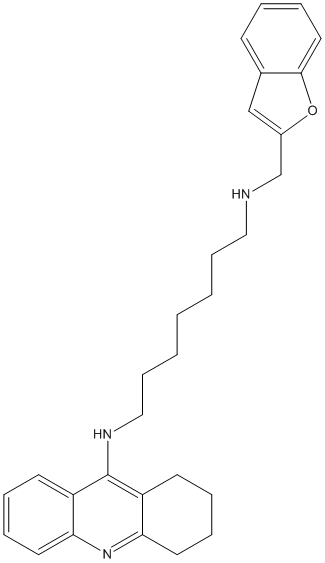
Tacrine-Benzofuran
compound able to reduce the hydrolytic activity of AChE, and potentially prevent amyloid production through the inhibition of BACE-1 activity || AChE Ki 0.00072 microM IC50 0.00086 microM; BChE IC50 0.00218; BACE-1 IC50 1.35 microM
General
Type : Multitarget, BACE1-inhibitor, Derivative of Tacrine, Acridine, Alkyl linked bis-ligand, Benzofuran
Chemical_Nomenclature : N-(1-Benzofuran-2-Ylmethyl)-N'-(1,2,3,4-Tetrahydroacridin-9-Yl)heptane-1,7-Diamine
Canonical SMILES : C1CCC2=NC3=CC=CC=C3C(=C2C1)NCCCCCCCNCC4=CC5=CC=CC=C5O4
InChI : InChI=1S\/C29H35N3O\/c1(2-10-18-30-21-23-20-22-12-4-9-17-28(22)33-23)3-11-19-31-29-24-13-5-7-15-26(24)32-27-16-8-6-14-25(27)29\/h4-5,7,9,12-13,15,17,20,30H,1-3,6,8,10-11,14,16,18-19,21H2,(H,31,32)
InChIKey : LJASCSHXLYLOCF-UHFFFAOYSA-N
Other name(s) :
MW : 441.60
Formula : C29H35N3O
CAS_number :
PubChem : 91864546
UniChem : LJASCSHXLYLOCF-UHFFFAOYSA-N
Target
Families : Tacrine-Benzofuran ligand of proteins in family
ACHE
BCHE
Structure :
4W63
Protein :
torca-ACHE
human-ACHE
human-BCHE
References (1)
| Title : Novel Tacrine-Benzofuran Hybrids as Potent Multitarget-Directed Ligands for the Treatment of Alzheimer's Disease: Design, Synthesis, Biological Evaluation, and X-ray Crystallography - Zha_2016_J.Med.Chem_59_114 |
| Author(s) : Zha X , Lamba D , Zhang L , Lou Y , Xu C , Kang D , Chen L , Xu Y , De Simone A , Samez S , Pesaresi A , Stojan J , Lopez MG , Egea J , Andrisano V , Bartolini M |
| Ref : Journal of Medicinal Chemistry , 59 :114 , 2016 |
| Abstract : |
| PubMedSearch : Zha_2016_J.Med.Chem_59_114 |
| PubMedID: 26632651 |
| Gene_locus related to this paper: torca-ACHE |